
Steps companies can take to help safeguard patients and the pharma supply chain.

Steps companies can take to help safeguard patients and the pharma supply chain.
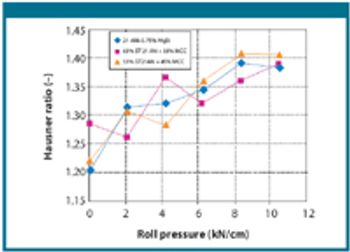
The authors studied the behavior of anhydrous lactose and the combination of anhydrous lactose and the combination of anhydrous lactose with microcrystalline cellulose on a pilot-scale roller compactor.

On March 19, 2009, IMS, the leading provider of market research for the pharmaceutical and healthcare industries, reported that annual sales of prescriptions in the US grew 1.3% from 287.6 billion in 2007 to 291.5 billion in 2008.

Also, Hospira to reduce workforce; WuXi AppTech makes senior appointments; more...

In a case decided on Mar. 20, 2009, the US Court of Appeals for the Federal Circuit invalidated a US Patent and Trademark Office (PTO) Final Rule that governed the number of applications that parties may file to seek continued examinations of patent applications.

President Barack Obama signed the fiscal year 2009 Ombnibus Appropriations Act on Mar. 11, 2009, giving the US Food and Drug Administration $2.6 billion. The funding can be used through the end of the fiscal year, Sept. 30, 2009.

The US Pharmacopeial Convention (USP) and the National Institute for the Control of Pharmaceutical Biological Products (NICPBP), China's agency for overseeing the quality of large- and small-molecule drugs, signed a memorandum of understanding (MOU) to bolster the quality of medicines in China and in the countries that buy Chinese drug products, including the United States.

Also, Genzyme receives warning letter; Mesa Laboratories appoints John J. Sullivan CEO and a member of the board of directors; more...
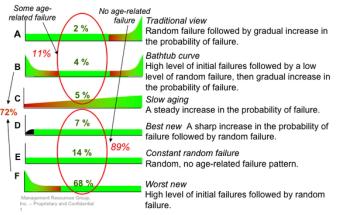
Time-based maintenance programs preserve the equipment but usually not its function, and they do not mitigate equipment failure for the balance of a machine's life cycle.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the February 2009 edition from PortaFab and Sterling.

The spotlight on the biopharmaceutical industry is intensifying, as recently evidenced by Pfizer's (New York) ongoing acquisition of Wyeth (Madison, NJ), which was initiated partly to reduce the former's dependence on small-molecule drugs.

2009 Pre-Interphex Showcase: Cleanrooms

Also, Penn Pharma to expand; stem cell research funding ban lifted; Bristol-Myers Squibb made senior appointments; more...

Merck & Co. (Whitehouse Station, NJ) and Schering-Plough (Kenilworth, NJ) completed a definitive merger agreement under which Schering-Plough stockholders will receive $23.61 per share.

President Obama nominated Kansas Governor Kathleen Sebelius as his new Secretary of Health and Human Services (HHS).

President Obama's Fiscal Year 2010 (FY 2010) budget includes support for a regulatory pathway for follow-on biologics.

On March 3, the US Food and Drug Administration released a draft guidance for Industry entitled "Clinical Pharmacology Section of Labeling for Human Prescription Drug and Biological Products: Content and Format."

In a press release dated Feb. 25, 2009, the US Food and Drug Administration charged that Ranbaxy Laboratories's (Gurgaon, Haryana, India) Paonta Sahib facility falsified data and test results in approved and pending drug applications.

Also, Schering-Plough's vaccine unit, Nobilon, formed an agreement with the World Health Organization; Ore Pharmaceuticals named president and CEO; more...

Ultra high performance liquid chromatography is advantageous in a contract laboratory because it is faster, more sensitive, and relies on smaller volumes of organic solvents than HPLC.

USP's Stage 2 heparin monograph revisions address identification, potency, and impurities.
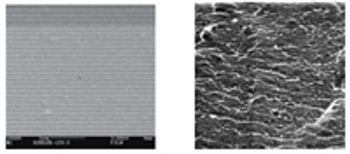
The authors investigate the effects of a polyethylene glycol plasticizer and water on cellulose acetate film properties.

Broader disclosure of drug prices and conflicts of interest are central healthcare reform issues.

Industry has changed, but its basic tenets have not. INTERPHEX's RJ Palermo discusses a 7-step process to keep pharma moving forward.

The source of a problem reveals itself after some investigation, or it may crash down on you.