Aseptic/Sterile Processing
Latest News

Latest Videos

More News

Shifting to automated, closed modular manufacturing systems is growing increasingly crucial for biopharmaceutical production.
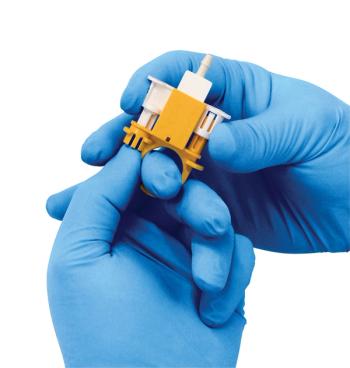
CPC’s MicroCNX ULT Series represents the first aseptic microconnector that can be fitted directly into freeze cassettes for GCT production.

The growing use of continuous manufacturing in the pharmaceutical industry merits a review of the application of dry cleaning and sanitization methods, especially for non-sterile oral solid drug product manufacturing of dry powders.

US Specialty Formulations plans to invest $15 million to expand biopharma operations at its Allentown, Pa.,facility.

BioIVT opened new cleanroom manufacturing space, using Germfree’s technology to boost its capabilities for cell and gene therapy development.

This $4.1 billion investment to build a second fill/finish manufacturing facility in Clayton, N.C., boosts Novo Nordisk's current 2024 investments in production to $6.8 billion.

In this episode, Hanns-Christian Mahler from ten23 Health chats about the advances in fill/finish.

Leveraging automation advancements can help improve efficiency in aseptic processing and speed up commercialization of critical drug products.

Improved efficiencies and reduced costs are clear advantages of automating drug manufacturing processes.

At INTERPHEX 2024, Pharmaceutical Technology® chatted with Dennis Powers from G-CON Manufacturing about podular cleanroom options and the benefits they offer for manufacturers.

Mary Van Gaasbeck, technical services specialist, LS Equipment and Services, at STERIS Life Sciences, notes that automation is key when it comes to effective sterile powder transfer of parenteral drug products.

At INTERPHEX 2024, Pharmaceutical Technology sat down with Christa Myers of CRB Group and Nadiyra Walker Speight of Fujifilm Diosynth Biotechnologies to discuss implementation of Annex 1.

The airlock solution for cleanrooms offers contamination controls for a highly controlled environment.

CDMO White Raven aims to reduce contamination risk and gain the capability to handle multiple product formats with the installation of Cytiva’s SA25 Aseptic Filling Workcell.

Misalignment around liquid filtration requirements and contamination control assurance still persist despite the revisions to Annex 1.

IREM can be used for effectively assessing and mitigating risks and improving the overall sterility assurance level in all types of aseptic processing lines.
Managing the intricacies of sterile product development is imperative for successful and compliant outcomes.

Novo Nordisk to Make Multi-Billion Dollar Investment in Expansion of Production Facilities in France
Novo Nordisk will invest more than DKK 16 billion (US$2.3 billion) to expand its production facilities in Chartres, France.

Washed seals save drug makers time and money.

EMS is a leader in the cleanroom industry, delivering complete cleanroom monitoring and control solutions, services to customers, and ensuring quality by design from inception to process control.

Environmental monitoring data can help keep sterile environments sterile.

To overcome the challenges of the widening range and scope of products that require aseptic processing and the evolving regulatory landscape in this field, companies should deepen their knowledge base on best practices.

Data from environmental monitoring can assist in keeping sterile environments sterile.

Manufacturers must figure out how flexible they need to be to meet the numerous new requirements of the changing therapeutic and regulatory landscapes.

Aseptic techniques must be practiced throughout all stages of biologics production.