Manufacturing, Cell Therapies
Latest News

Latest Videos
More News

Although specifics about the milestone were not immediately provided, Scribe is eligible to receive more than $1.2 billion for certain research, development, regulatory, and commercial benchmarks.

The transaction adds a pipeline of early-stage drug candidates against high-value targets to Telix’s portfolio, along with other novel targets currently in the discovery stage.

The Thousand Oaks, Calif., cell therapy manufacturing facility now houses new production suites, updated development labs, and more after expansion.

Manufacturers of five autologous or matched allogenic cell therapy products have selected TrakCel's IT platform, OCELLOS, to orchestrate the administration of these therapies, which are approved or expected to be approved in 2024.

The new Institute for Cell Therapy Discovery and Innovation will utilize expertise to develop cell therapies for cancer, autoimmune diseases, and infections.
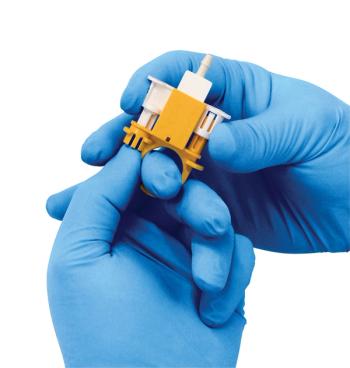
CPC’s MicroCNX ULT Series represents the first aseptic microconnector that can be fitted directly into freeze cassettes for GCT production.

The two companies are partnering to provide logistics and manufacturing services to biotechnology and pharma companies.

Lonza signed a long-term supply agreement to manufacture CASGEVY (exagamglogene autotemcel) for Vertex at its facility in Geleen, the Netherlands, and plans to expand manufacturing to Portsmouth, NH, in the United States.

The next-generation IRO platform was found to significantly outperform the legacy Prodigy system, showing improvements in cell growth, CAR+ cell yield, and overall efficiency.

Viral vectors and other complex biologic modalities require more specificity and higher sensitivity to detect and distinguish contaminants.

Rentschler Biopharma has expanded its service offerings at its Stevenage, UK, site with a new lentiviral vector manufacturing toolbox.

BioIVT opened new cleanroom manufacturing space, using Germfree’s technology to boost its capabilities for cell and gene therapy development.

Innovative solutions are making personalized cell and gene therapies accessible to all.

PluriCDMO, Pluri's newly launched CDMO division, has been selected by Kadimastem to manufacture two novel cell therapy product candidates.
No timetable was given for the length of the planned evaluation or when data would be available, but Kite intends to use the information to expand its manufacturing options.

Lonza’s Joe Garrity and Jerry Jiang discuss the importance of not only automating CGT manufacturing, but also standardizing across processes.

Lonza’s Joe Garrity and Jerry Jiang discuss the latest trends and challenges in commercializing new CGTs.

CGT Catapult and CATTI aim to standardize advanced therapy manufacturing with new aligned training standards.

CGT Catapult will implement Cellular Origins' robotic platform in its Stevenage, UK, site to establish automated CGT manufacturing.

With this acquisition, Merck KGaA will have the capability to offer integrated services for viral vector manufacturing.

Regeneron's Sven Moller-Tank, PhD, director of Viral Delivery Technologies, Regeneron Genetic Medicines, discusses the company's work in using bispecific antibodies for redirecting AAV target specificity.

Experiments conducted by the downstream technology team at Spark Therapeutics involving metal ion-containing additives showed improved capsid clearance in AAV production.

ProPharm and PBL have introduced a fully automated, enclosed cell factory manufacturing device.

A new draft guidance issued by FDA covers human- and animal-derived materials used in the manufacture of advanced therapy medicinal products.

In the new draft guidance, FDA provides recommendations on cell safety testing of human-sourced allogeneic cells for sponsor companies.