
The generic-drug manufacturer Actavis reached an agreement on a consent decree of permanent injunction with the US Food and Drug Administration regarding the company?s US subsidiary Actavis Totowa LLC.

The generic-drug manufacturer Actavis reached an agreement on a consent decree of permanent injunction with the US Food and Drug Administration regarding the company?s US subsidiary Actavis Totowa LLC.

A California jury ordered Pfizer (New York) to pay $38 million to the Ischemia Research and Education Foundation (IREF), a medical-research nonprofit group, for stealing trade secrets to develop its "Bextra" analgesic.

Outsourcing sterile manufacturing involves an integrated approach in product life-cycle management.
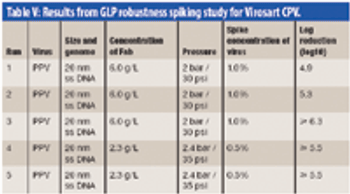
The authors examine the challenges of integrating a large-scale chromatography and nanofiltration process for purification of a polyclonal antibody.

Although its domestic market is on the rise, Pakistan's market conditions have not proved welcoming enough to keep foreign investors.
Editors' Picks of Pharmaceutical Science & Technology Innovations

The incoming administration has renewed hope for stem cells, but less adequate copycats may follow.

After a year of increased attention on the pharmaceutical supply chain in Asia, what will be the region's short- and long-term role? This article contains bonus online-exclusive material.

A book explains the many pharmaceutical applications of polymers from natural sources.

Brief pharmaceutical news items for January 2009.
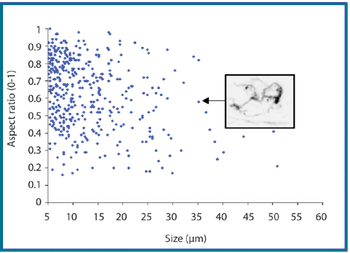
The authors describe a novel analytical approach that uses the shape-analysis capabilities of MFI to detect and enumerate silicone oil microdroplets in protein formulations that also contain aggregates of similar size and in a similar concentration.
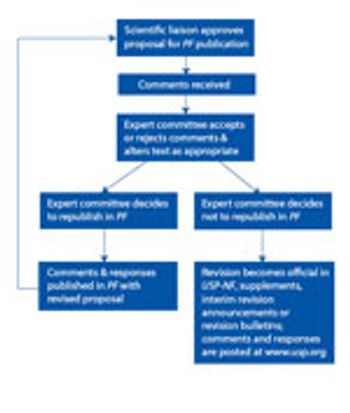
The USP public-comment process exists for a reason. Industry needs to take advantage. This article contains bonus online-exclusive material.
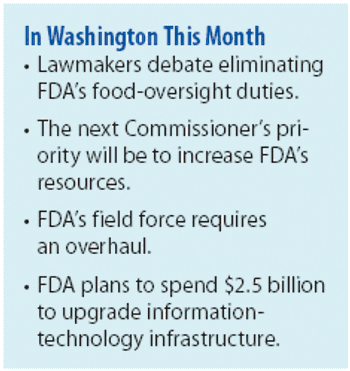
Will more resources and new leadership fix FDA, or is a major overhaul in order?

The authors propose an approach for qualification-target selection and show how it can be applied to blister-filling and packaging systems.

Instead of hampering progress, cost pressures might inspire Big Pharma to get creative.

Readers give advice on their best approach to handling (batch) rejection

The biotechnology sector is occasionally described as a rainbow, with each sub sector having its own colour. But what do the different colours of biotechnology have to offer the pharmaceutical industry?

Conflict between departments costs money, wastes time and hinders strategy implementation. But we can reduce it and manage it if we understand it, says Dr Brian D. Smith.

A new year, all things being equal, is a time of looking to the future with positive intent and traditional bonhomie. At the very least, it suggests new beginnings. As we enter 2009, however, the world is moving into a major recession and things are far from equal.

Creating better pharmaceutical and medical products with packaging partnerships.
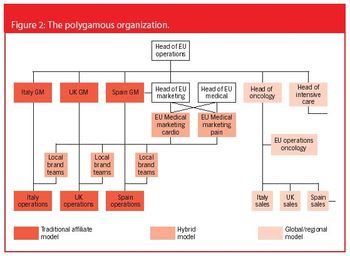
Paul Gardiner and Andrea Sobrio explain why most companies need 'organizational polygamy' to maximize profit and best serve their customers.

Hot-melt extrusion offers many advantages compared with conventional solid dosage form manufacturing, and has consequently received considerable attention from both the pharmaceutical industry and academia as a novel drug delivery technology. The possibility of forming solid dispersions with improved bioavailability renders hot-melt extrusion an excellent alternative to other conventionally employed techniques.

Reducing ecological footprint and achieving more sustainable production of pharmaceuticals could help create a better future.

Also, Crucell and DSM announce deals with GSK, Talecris, and CSL; Nobel Prize winner Luc Montagnier joins Viral Genetics; more...

The US Food and Drug Administration dedicated historic Building One at the White Oak Federal Research Center in Silver Spring, Maryland.