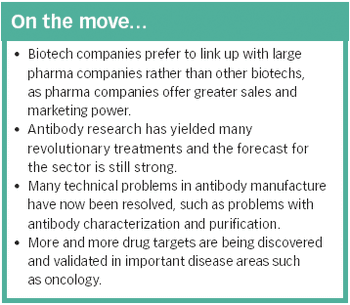
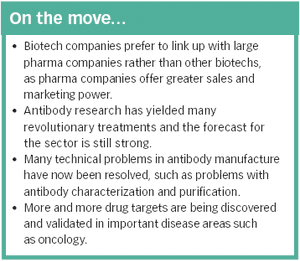
The identification of an increasing number of drug targets coupled with advances in manufacturing technology could lead to many more blockbuster therapeutic antibodies.

The identification of an increasing number of drug targets coupled with advances in manufacturing technology could lead to many more blockbuster therapeutic antibodies.

The biotechnology sector is occasionally described as a rainbow, with each sub sector having its own colour. But what do the different colours of biotechnology have to offer the pharmaceutical industry?

Hundreds of pharma–biotech deals have been announced during 2008 and many of these probably began at biopartnering conferences. Susan Aldridge examines some of this year's big buyouts and mergers.

There are many challenges upstream and downstream in manufacturing a biotech drug.

In the popular view of nanomedicine, miniature robots equipped with a set of tools will one day patrol the inside of the body, cleaning up atheroslerotic plaque, zapping cancer cells, and generally performing repair and maintenance.

Researchers at Children's Hospital Boston (MA, USA) recently announced another group of stem cells that can produce cardiomyocytes (heart muscle cells). It seems hardly a month goes by without some kind of discovery in the fast-moving world of stem cells.

... profit margins in ophthalmology are very attractive and the market is increasing as the incidence of eye disease grows with the ageing population.

Once famous for coal and steel, Wales now aims to make its mark in the life sciences sector. The recent BioWales 2008 (UK) conference demonstrated how much the region has progressed, thanks to the support of the Welsh Assembly government, the EU and the enthusiasm of the Welsh biotech community. The country's bioscience sector, which comprises approximately 200 companies, grew by 18% last year and is now worth £1.3 billion (€1.6 billion) in annual turnover, employing 15–20000 people.

Insulin is one of the world's oldest and most well-known biological drugs, and the need for it is not going to go away as the number of patients diagnosed with diabetes continues to increase. A wealth of clinical evidence shows that good, long-term glucose control in diabetes is key to avoiding complications such as kidney disease, blindness and heart problems.

The amount of water used by industry, including pharma and biotech manufacturing, amounts to 23% of the world's supplies

My personal experience of lab automation is limited to supervising a peptide synthesiser back in the late 1980s. The machine was eye-wateringly expensive - but it was soon paying its way in terms of productivity and research publications. So if I were a stake-holder in a company pondering whether to invest in a well-designed gadget that could automate a routine operation, I'd say: 'go for it'.

Deciding where in the world to locate a new plant is a key decision for any pharma or biotech company - and there has never been more choice. Europe and the US now compete with the Far East and India, and what about the new EU states? Might Lithuania or Estonia turn out to offer advantages compared with France or Germany when it comes to finding the best place to take a new drug forward to the market place?

While it's unlikely that Al Gore's Nobel Peace Prize will gain him the US Presidency, the award has put global warming firmly in the spotlight (as if it wasn't there already). Reducing its carbon footprint is among the many goals Big Pharma has for the coming year - and beyond. Many energy consumption issues are common to all industries and individuals, such as putting curbs on business travel, bringing renewable energy sources on board and switching off lights in unoccupied rooms, but there are other issues that are specific to our industry. For example, asthma inhalers emit greenhouse gases and many pharmaceutical syntheses use toxic solvents.

In recent years, Big Pharma companies have shown increasing interest in setting up manufacturing in India and the Far East. The advantages of this outsourcing trend are obvious - decreased costs, coupled with ever increasing standards of operation. However, as the technological and quality of life aspirations of these new locations increase, the attractive cost advantages could dwindle and cause pharma companies to look elsewhere for manufacturing locations. Perhaps, they will consider Africa.

Vaccines are needed against old and new infectious disease threats - polio and other childhood illnesses, bioterrorism and pandemic flu. They are also emerging for cancer immunotherapy and for treating addiction. While vaccines are among some of the most successful biotech products, their large-scale manufacture involves some special demands, such as maintaining a good working cell bank and gearing up for production on an 'as needed' basis.

Last year, a monoclonal antibody, TGN1412, led to potentially fatal adverse effects in a small group of Phase I volunteers in London. In the wake of this incident, EMEA has drawn up new guidelines that could lead to demands for more data on novel biologics. They may have implications for both manufacturing and clinical trials of biopharmaceuticals that are considered to pose a high risk to patients.

Getting from a cell culture to a purified biotech product is a demanding exercise involving many operations. Increasing productivity in the upstream part of biotech production is placing new demands on the purification process, which may lead to adopting new technologies.

... the biotech industry could make characterization of its products easier by paying more attention to downstream processing and purification issues, creating a cleaner product that is easier to identify.

It has been a long time coming, but stakeholders in the US are now seriously debating a route to market for cheaper copies of biopharmaceutical drugs. The European Agency for the Evaluation of Medicinal Products (EMEA) has led the way on this issue by publishing clear guidelines on what companies must do to get their versions of drugs such as erythropoietin (EPO), an advanced treatment for anæmia, and similar products approved.

Green chemistry involves redesigning processes so that more of the raw material ends up in the product, rather than as waste...

... there is a huge manufacturing challenge involved in bringing cell therapies (especially stem cell therapies) from the lab bench into the clinic.

Disposables simplify the flow of people and materials through a facility, decreasing both its footprint and amount of equipment needed, as well as minimizing upfront capital costs.

Brain and heart broth is still used in path labs, but, when it comes to biopharmaceutical manufacturing, cells are having to learn to do without animal components whenever possible.

There are currently several hundred biotech-based drugs in clinical trials, representing around a quarter of all drugs in development - a proportion that looks set to increase.
Published: May 1st 2009 | Updated:

Published: May 1st 2008 | Updated:

Published: April 1st 2008 | Updated:

Published: October 1st 2007 | Updated:

Published: May 1st 2007 | Updated:

Published: January 1st 2008 | Updated: