
Pharmaceutical training programs are enhanced by integrating ICH quality risk management considerations.

Pharmaceutical training programs are enhanced by integrating ICH quality risk management considerations.

This paper reflects upon the past 15 years of experience in the application of QRM and KM within the pharmaceutical GMP environment.

It is important to understand the differences between risk-based decision making and other decision making in a pharmaceutical quality system.

This article explores the emergence of subjectivity in ICH Q9 (R1).

Quality Quartets may be used to achieve knowledge-driven, risk-based approaches to commissioning and qualification that are consistent with ICH Q9(R1) principles.

This paper explores the relationship between investing in pharmaceutical manufacturing risk reduction and meeting business objectives.

A thoughtfully constructed QRM Master Plan translates the strategy and enables a risk-based approach.

The ICH Q9 revision clarifies QRM implementation for the pharmaceutical industry and expands descriptions of methodology, formality, and risk-based decision making to provide the industry with a solid base from which to build their own QRM programs.

More attention should be given to how expert opinions and judgments are elicited for reducing uncertainty in quality risk management and risk-based decision making.
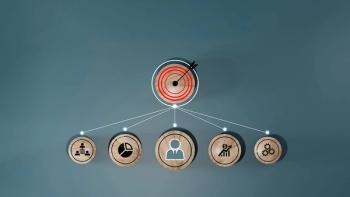
A control strategy can function as a powerful “hub” to gain a comprehensive understanding of the consolidated set of controls that are necessary to ensure consistent delivery of quality product.

Recent research and perspectives shed light on an opportunity to better connect risk and knowledge through improved integration of systems.

The degree of formality in a risk management process should be customized to the organization’s particular needs and the risks involved.

Using the four-phased method to assess QRM can ensure continual improvement and that regulatory requirements are met.

Quality Quartet Registers connect Quality Quartets to their process system or unit operation “parent.”