
Sanofi-aventis introduced this week its Biolaunch project at its Vitry-sur-Seine, France, pharmaceutical production site.

Sanofi-aventis introduced this week its Biolaunch project at its Vitry-sur-Seine, France, pharmaceutical production site.

This week, pharmaceutical industry regulators and manufacturers moved quickly to address public health concerns regarding the outbreak and spread of H1N1 virus infection (swine flu). The following is an overview of key developments.

Pharmaceutical companies must make bold moves and "step outside of their sector" if they are to survive.

Strong growth in biopharmaceuticals bodes well for contract manufacturing, but the perils and the promises of pipelines remain.

The president of BIO proposes the ingredients needed for industry growth.

Process steps, GMP documents, a purification vessel, and validation seem to disappear.
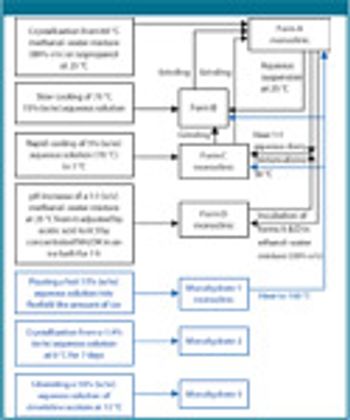
The authors describe the importance of a rapid and an abbreviated screening strategy by initial solvent screening in 20-mL scintillation vials.

The Japanese government is eager to jumpstart its generic-drug market, but changes must come first.

The GDP committee of IPEC–Europe is trying to seal one more broken link in the supply chain. This article contains bonus online-exclusive material.

Brief pharmaceutical news items for May 2009.
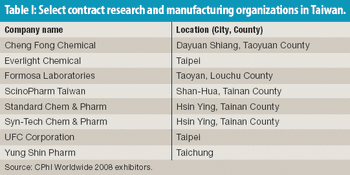
Taking a cue from its electronics industry, Taiwan is seeking to put its biotechnology and pharmaceutical industries on the map. An interactive map shows pharmaceutical activity in Taiwan.

Government funding is slated to boost comparative studies of prescription drugs.

Manufacturers of therapeutic monoclonal antibodies consider new paradigms in purification technologies.

Products at INTERPHEX focused on protection, compliance, and deterring counterfeiting. This article contains bonus online-exclusive material.

The organizations' presidents discuss market exclusivity, approval processes, and pending legislation.

Technology can solve enterprise-level problems.

Editors' Picks of Pharmaceutical Science & Technology Innovations
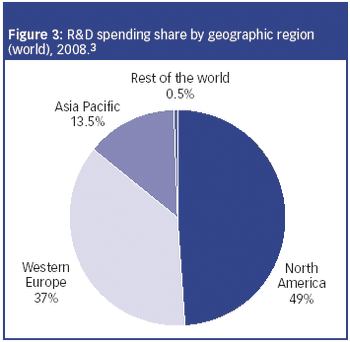
The CRO market is experiencing two-tiered growth: firstly from pharmaceutical companies seeking to lower fixed costs by outsourcing clinical research to CROs; and secondly, from biotechnology and specialty pharmaceutical companies that lack the infrastructure to conduct trials.

Bulk Inspection of Tablets-Symetix Application Note
The identification of an increasing number of drug targets coupled with advances in manufacturing technology could lead to many more blockbuster therapeutic antibodies.

A novel cleanroom apparel design incorporates modern concepts to help minimize contamination. Take a tour of the design.
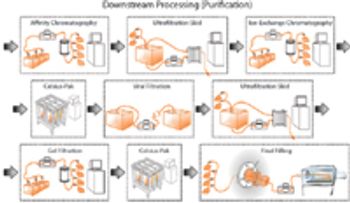
The authors discuss current and future disposable technologies and outline the validation and qualification steps that would be required for a possible disposable process stream.

The authors review the role of automation in aseptic processing and describe their experience in implementing advanced technologies, including the use of isolators and robotics.

The author provides a history of the information chapter USP ‹1211› "Sterilization and Sterility Assurance of Compendial Articles," from the early 1900s to the current version.
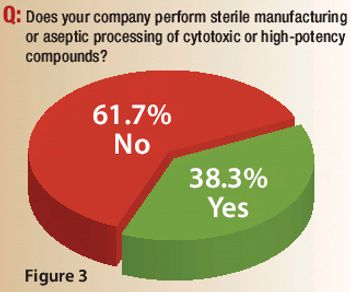
A Pharmaceutical Technology survey examines capacity expansions, outsourcing practices, innovation levels, and the role of quality by design in sterile manufacturing and aseptic processing.