
CROs and CMOs expand to gain a piece of the market for clinical trial materials.

CROs and CMOs expand to gain a piece of the market for clinical trial materials.

It can take a lot of work to make sure nothing happens.

Short-term problems in software or hardware lead to long-term manufacturing troubles.
Debates about science, manufacturing, and European regulations will shape the approval process for follow-on biologics in the United States.

Editors' Picks of Pharmaceutical Science & Technology Innovations
Despite its shrinking domestic economy, Ireland is determined not to let its pharmaceutical industry fade into the shadow of global recession.

Agency officials and manufacturers anticipate stricter enforcement of drug safety and quality.

Brief pharmaceutical news items for June 2009.

FDA leaders explain the purpose and plan for ICH's three quality guidelines.

In 2005, a small delegation (myself included) of the European Fine Chemicals Group (EFCG) met with the deputy head of the cabinet of Commissioner Kyprianou (the then Commissioner responsible for health and consumer protection). Our mission was simple - we were there to raise a red flag.
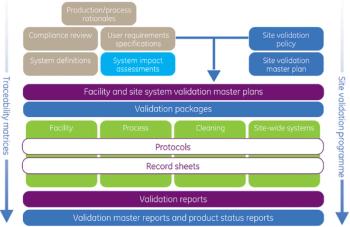
Despite the fact that regulatory compliance is fundamental to the pharmaceutical industry, too many manufacturers lose millions of euro in revenue because of poorly or incorrectly validated facilities. With increasingly demanding global regulations and guidance, rising manufacturing costs and dwindling product portfolios, it is vital to achieve efficient and effective compliance of facilities, processes and equipment to retain market competitiveness.

Today, approximately 1.5 million counterfeit medicine packs enter the legal supply chain each year - in other words, one pack in every 20000 is counterfeit.

The United States Department of Health and Human Services (HHS) this week placed an initial order with Sanofi Pasteur (Lyon, France) for a vaccine to fight influenza A (H1N1) infection.

Novartis (Basel) signed an agreement that grants the company an exclusive option to acquire Elixir Pharmaceuticals (Cambridge, MA) upon the successful completion of a Phase IIa clinical study of Elixir's lead oral ghrelin antagonist, which is now the subject of preclinical studies.

The European Federation of Pharmaceutical Industries and Associations (EFPIA) announced a pilot program, to launch in Sweden later this year, that will focus on coding and identification solutions.

Also, Johnson & Johnson acquires Cougar Biotechnology; NIH launches program for rare and neglected diseases; PPD restructures leadership positions; more...

The US Pharmacopeia will release for public comment a new USP <231> Heavy Metals general chapter. Is your laboratory ready to handle the changes? Find out at Pharmaceutical Technology's June 4 webinar. And check out our interactive metals and limits table.

Pfizer's Pharmacia unit may be ordered to pay nearly $212 million as a result of a February 2009 Wisconsin court ruling that found the company guilty of violating the state's Medicaid fraud statute 1.44 million times.

Also, Oxford BioTherapeutics forms drug development pact with GSK; Avila Therapeutics names CEO; more...

The World Health Organization's Ad Hoc Policy Advisory Working Group on Influenza A (H1N1) Vaccines issued an update on May 18, 2009, on the production status of seasonal and A (H1NI) vaccines.

Last Monday, Billy Tauzin, president and CEO of the Pharmaceutical Research and Manufacturers of America (PhRMA), voiced the group's support for President Obama's efforts to reform the nation's healthcare system.

The US Food and Drug Administration intends to automate and enhance the safety of the pharmaceutical supply chain by establishing electronic pedigrees (ePedigrees) for drug products.

Automation has been part of pharmaceutical processing for some time, but robotics have not yet found wide acceptance among drugmakers.
PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the May 2009 edition from Continental Disc and Filimatic.

Sanofi-aventis announced this week that it plans to construct a new vaccine-manufacturing center in Neuville-sur-Saône, France.