
Brief pharmaceutical news items for February 2009.

Brief pharmaceutical news items for February 2009.

The author defines sanitizers, disinfectants, and antibiotics, and examines the question of whether the rotation of disinfectants is scientifically warranted.
The pharmaceutical majors forward projects in biocatalysis, solvent replacement, and other approaches in green chemistry.

Editors' Picks of Pharmaceutical Science & Technology Innovations
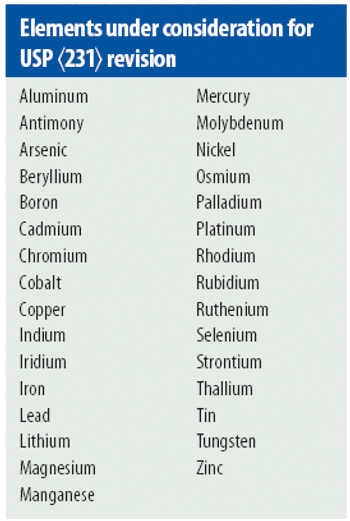
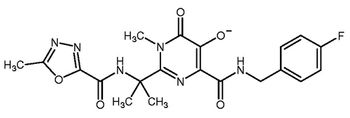
USP <231> Heavy Metals is transitioning toward the incorporation of modern quantitative technologies, but there is still much to be resolved.

To manage risk properly, industry must understand what it is and how to assess it

A recent reader poll focused on predictions for the industry's future.

The Romanian pharmacetuical market has grown significantly in recent years, but with stagnation on the horizon, a path forward is needed.

Efforts to cut healthcare outlays will focus on drug costs, despite a drop in prescription sales.

A new book inspires readers to seek ways to apply NMR spectroscopy to their own purposes.

FDA's role should not be overlooked as it has been in years past.
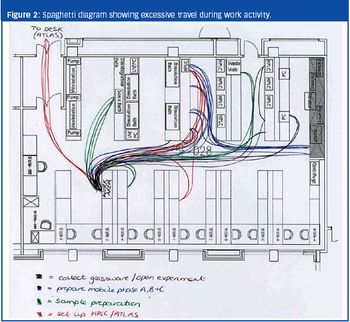
Lean Sigma approaches can reduce waste, cost, cycle time and variability in outputs.

Pfizer is no normal company. As recently as 2007 it was still the industry's largest player with $47.5 billion (36.8 billion euro) coming in from pharma sales alone.

A discussion of the basic principles of immunity and the nature of antibodies.

During the last 20 years, pharmaceutical R&D spending has increased 15-fold while new product approvals have increased only 0.70-fold.

The American Society for Quality (ASQ), a membership organization that focuses on quality training in several industries including pharmaceuticals and healthcare, has adopted a new certification for good manufacturing practice (GMP) professionals.

Also, UCB will divest certain business in emerging markets to GSK; the KineMatik Group named Michael G. Jarjour president and CEO; more...

Pfizer will purchase Wyeth (Madison, NJ) under a definitive merger agreement approved by both companies' boards of directors.

Also, Elan explores strategic alternatives; NanoGuardian appoints John D. Glover to lead its Security Advisory Board; more...

Eli Lilly and Company has reached resolution regarding its previously reported government investigation into the company's US marketing and promotional practices for "Zyrexa" (olanzapine), an antipsychotic drug, thereby ending a government investigation started in 2004.

Last week, the US Food and Drug Administration issued a draft guidance for industry, Standards for Securing the Drug Supply Chain–Standardized Numerical Identification for Prescription Drug Packages, and launched a pilot program to help protect the pharmaceutical supply chain.

The US Department of Health and Human Services's Biomedical Advanced Research and Development Authority has awarded Novartis a contract valued up to $486 million over eight years to support the design, construction, validation, and licensing of a US cell-based influenza vaccine manufacturing facility in Holly Springs, North Carolina.

The US Food and Drug Administration issued its Draft Guidance for Industry on Submission of Laboratory Packages by Accredited Laboratories.

One relatively new dosage form is the orally dissolving strip, a thin film formulated with hydrophilic polymers that rapidly dissolves on the tongue.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the January 2009 edition from Clark Solutions and Neopac.