
Also: Sanofi-aventis acquires BiPar Sciences; FDA issues Warning Letter to Chinese heparin manufacturer; Halo Pharmaceutical appoints chief scientific officer; more...

Also: Sanofi-aventis acquires BiPar Sciences; FDA issues Warning Letter to Chinese heparin manufacturer; Halo Pharmaceutical appoints chief scientific officer; more...

Pharmaceutical giants GlaxoSmithKline (GSK) and Pfizer announced on April 16, 2009 that they are joining forces to create a new HIV-focused drug company.

The United States Pharmacopeial (USP) Convention signed a memorandum of understanding (MOU) with the Federal Service on Surveillance in Healthcare and Social Development of the Russian Federation (Roszdravnadzor) last week in Moscow.

Last week, Johns Hopkins Medicine (JHM) adopted a new policy to protect patients by limiting the influence of pharmaceutical marketing on faculty and physicians? decisions.

Also, Sanofi-aventis acquires Medley and Laboratorios Kendrick; Eli Lilly's Cook to retire from board; more...

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the April 2009 edition from Buzdar and Kaeser.

ATSM and ICH approaches, in place of traditional qualification or integrated commissioning and qualification (C&Q) using impact assessment, can make projects and processes more efficient and help facility owners and designers ensure compliance, quality, and safety when defining acceptance criteria for their critical process systems and equipment.

Many drugmakers have begun to evaluate and improve their manufacturing operations to become more economically and environmentally sustainable.

Pfizer outlined the progress of its late-stage pipeline last week and announced this week plans to separate its research activities into two organizations, one focused on small molecules and the other on biologics, pending the closure of its pending acquisition with Wyeth.

The Pharmaceutical Research and Manufacturers of America (PhRMA) elected David Brennan, CEO of AstraZeneca, as board chairman.

Also, Genzyme and Bayer HealthCare form agreement; FDA releases draft guidances; TransMolecular appointed Robert Radie president and CEO

2009 Post-Interphex Showcase: Cleanrooms

Although industry is tightening its belt, contract manufacturers across Europe are actually making out quite well by taking on additional projects and new roles.
Editors' Picks of Pharmaceutical Science & Technology Innovations
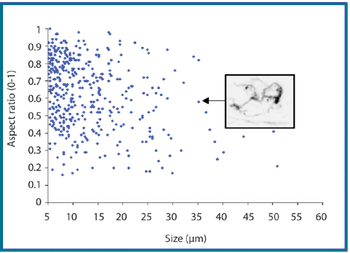
The authors describe a novel analytical approach that uses the shape-analysis capabilities of MFI to detect and enumerate silicone oil microdroplets in protein formulations that also contain aggregates of similar size and in a similar concentration.

Senator Charles Grassley (R-IA) sent a letter to Frank Torti, acting commissioner of the US Food and Drug Administration, to express concern about a memo that Torti sent to agency staff.

Also, SOCMA changes name; two FDA approvals; Biogen Idec names chief operating officer; more...

On March 26, the Michigan House of Representatives passed House Bill 4316, effectively repealing part of a 1996 law that provides drug companies immunity from liability lawsuits involving products that have been approved by the US Food and Drug Administration.

The United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA) seized nearly half a million pounds worth of counterfeit medicines on Mar. 26, 2009 in Middlesbrough, England, according to a MHRA press release.

Brief pharmaceutical news items for April 2009.

In a recent book, UK regulators explain how to establish a pharmacovigilance system.

FDA is poised to gain authority and resources to ensure the quality of food and drugs.
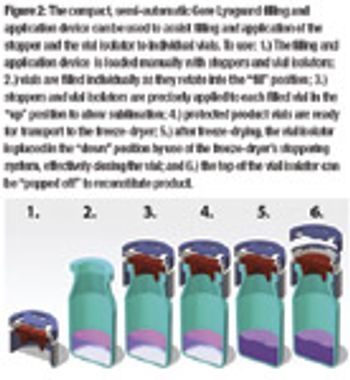
By reducing cycle time and implementing quality-by-design inspired engineering, advanced lyophilization systems are driving the industry toward greater efficiency and control.

Obama's cost-containment and science-innovation initiatives need to overlap.

President Obama's economic recovery plain includes goals such as reducing the number of uninsured citizens and improving the quality of healthcare.