
Increased treatment using combination therapies
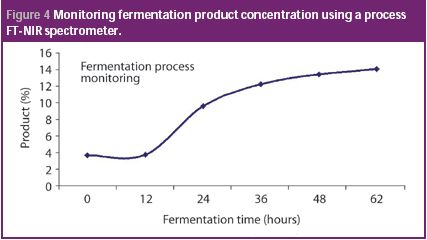
Quality by design and PAT approaches are increasingly being used for the biotech manufacturing of medicines. Complex manufacturing processes can not only be controlled using PAT principles, but optimized with respect to both product quality and economic value. This column describes how the fermentation process is often the first to benefit from this type of implementation.

Increased treatment using combination therapies

New York (Oct. 9)-Pfizer has positioned itself to enter the DNA-based vaccine market with its agreement to acquire UK-based PowderMed Ltd., which holds a pipeline of DNA-based influenza vaccines currently in clinical development for treating both seasonal and avian flu.

Rockville, MD (Sept. 28)-The US Food and Drug Administration has released the draft guidance for industry ?Characterization and Qualification of Cell Substrates and Other Biological Starting Materials Used in the Production of Viral Vaccines for the Prevention and Treatment of Infectious Diseases.?
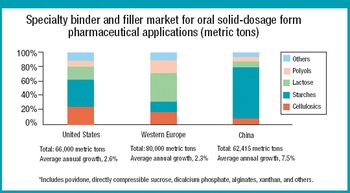
The specialty excipients market in the United States, Western Europe, and China is valued at nearly $800 million. The authors discuss the opportunities and challenges in these markets by examining the product mix, supply base, and preferred production methods.
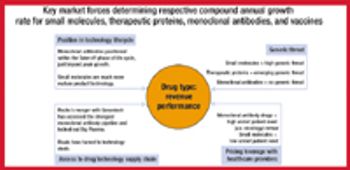
Biologics are forecast to account for roughly 60% of revenue growth through 2010 for Big Pharma as growth in small molecules slows. The author analyzes the factors driving demand and how the technology life cycles of these two sectors will affect market potential.

Plastic has emerged gradually as a viable packaging material, even for sterile products. Acceptance of plastics has been encouraged by blow–fill–seal (BFS) technology, which provides scientific and commercial benefits. BFS technology has, however, brought new challenges for formulation-development scientists. This article highlights the specific concerns for the development of sterile liquid products and the formulation strategies to address these concerns.

The authors investigated the influence of various particle size fractions of Tamarind seed polyose (TSP) on indomethacin (IND) release from matrix tablets. They assessed the TSP fractions for swelling, density, and flow properties and the IND matrix tablets for tensile strength, friability, and release profile. Release kinetics was evaluated using Higuchi and Peppas equations. The density and flow properties showed that the size fraction affects the suitability of TSP as an entrapment polymer. The release profile showed that the release of IND from TSP matrix is swelling dependent, thereby affecting the kinetics of release.
Protein formulation specialists have long sensed that something big could be just around the corner. Over the past few decades, countless companies have attempted to bring to market new protein therapeutics that offer improvements-be they more patient friendly, more effective, or easier to manufacture-over traditional formulations. Earlier this year, the launch of Pfizer's "Exubera" pulmonary insulin met this anticipation head on. The fast-acting, inhaled-powder form of recombinant human insulin brought hope to the millions of diabetic patients waiting for an alternative to injections.
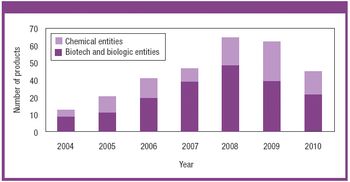
There is a growing need for patient-compliant dosage forms within the cancer therapeutics and biotechnology areas. Ease of administration, enhanced therapeutic efficacy, and reduced side effects are factors that differentiate drug delivery products from conventional dosage forms and provide a competitive advantage. This article reviews salient trends in the parenteral drug delivery sector within the realms of a changing regulatory environment, drivers to growth, and recent advances in this field. Challenges associated with bringing parenteral drug delivery concepts to commercialization are discussed.

In view of the nature of its complexity, it might be desirable to apply FDA's process analytical technology to lyophilization.
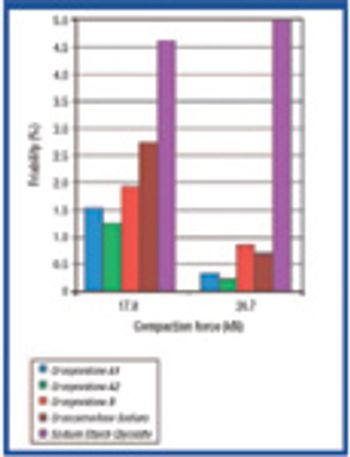
The increasing popularity of orally disintegrating tablets has led to growing interest in the advantages of superdisintegrants. This article presents some practical considerations in selecting these ingredients.

UK science has an outstanding record and we remain strong internationally in terms of achievement, productivity and efficiency.
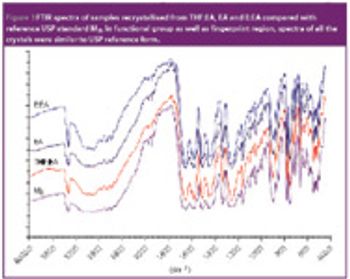
Mefenamic acid has variable bioavailability and tabletting issues because of its hydrophobic nature and poor material characteristics. Recrystallization of mefenamic acid was performed from three different solvent–solvent mixtures under differing conditions. The crystals obtained were screened for the existence of new crystal properties or polymorphic forms, then characterized further.
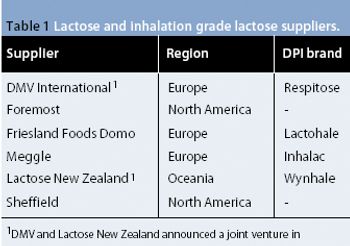
The presence of very low levels of residues (including solvents) in excipients is becoming an important issue for users, and the presence of very low levels of ?non-lactose? species in DPI lactose may pose challenges to suppliers and users.

High-containment manufacturing for highly potent active pharmaceutical ingredients (HPAPIs) represents a niche, but growing niche, in API manufacture. Strategies on serving this sector were discussed at a recent program, ?High-Potency Active Ingredients: Realizing the Opportunities,? organized by the Drug, Chemical, and Associated Technologies Association (Robbinsville, NJ) in conjunction with Pharmaceutical Technology.

The market potential for high-potency active ingredients will be influenced by growth patterns in the cytotoxic drug market. Strong growth is projected for cytotoxics through 2009, but the market then will see generic drug erosion, according to Sarah Terry Johnston, vice-president, healthcare, Datamonitor PLC.

As with pharmaceutical manufacturing as a whole, a risk-based approach is important in manufacturing highly hazardous or potent compounds. Industry groups are working with US Food and Drug Administration (Rockville, MD) to develop a baseline guide for a risk-management approach to determine containment controls required to minimize cross contamination.

Specialty Pharmaceutical Company Bentley Pharmaceuticals has entered into an agreement with Cardinal Health for the scale-up of clinical supplies for Bentley's intranasal insulin product candidate.

Baltimore, MD (Sept. 10)-Researchers at Johns Hopkins University have devised a new controlled-delivery system that applies an electrical pulse to release drug molecules, nanoparticles, biopolymers such as peptides and proteins, and protein assemblies such as viruses from thin fabricated gold electrodes. Developers hope the technique will allow biocompatible implantable chips for precisely dispensing small amounts of drug into the body.

Scientists from GlycoFi, Inc., a wholly owned subsidiary of Merck & Co, in collaboration with Dartmouth-Hitchcock Medical Center, have engineered yeast cells capable of producing a broad range of recombinant therapeutic proteins with fully human sugar structures (glycosylation).

The overall market size of the Southeast Asia region totals $7 billion, with a projected compound annual growth rate of about 13% through 2010.

For once, casting originators against generic players might end up strengthening the industry across the board.

As the pace of product development accelerates, the approach to dissolution-method development must advance beyond a manual method and an assay. A natural progression of the method-development process must include the transfer of the manual method onto automated instrumentation.

Potent-compound awareness training for operators is important to understand why the containment and controls are in place.

Biovail Corporation is demanding that the US Food and Drug Administration (Rockville, MD) enforce the proper criteria for determining bioequivalence of extended-release generic versions of bupropion products.