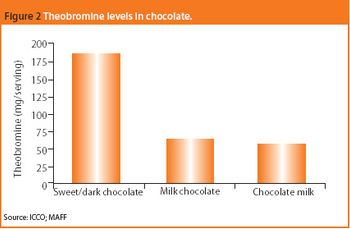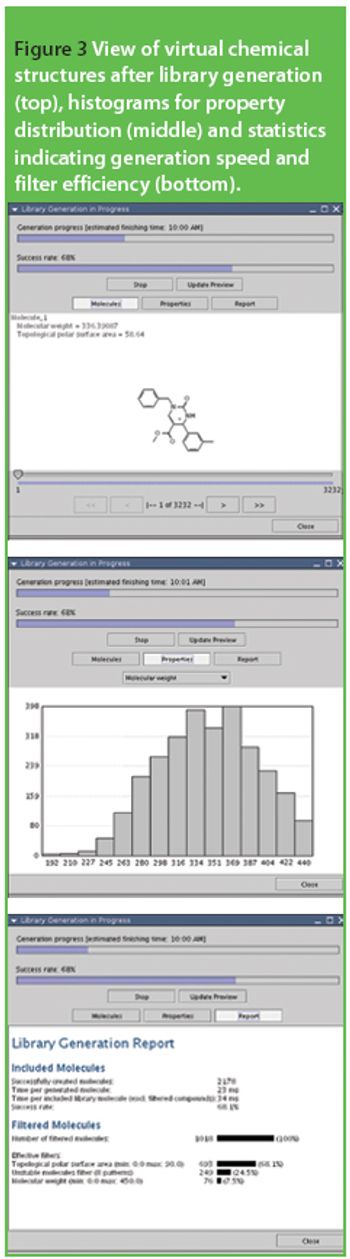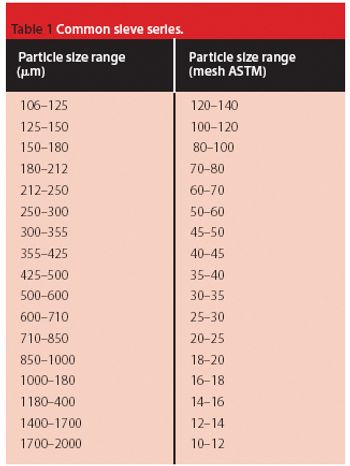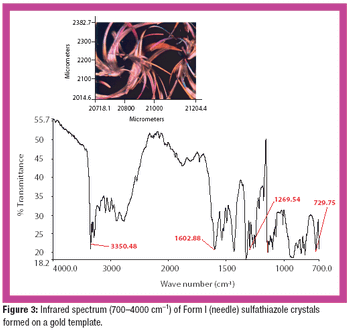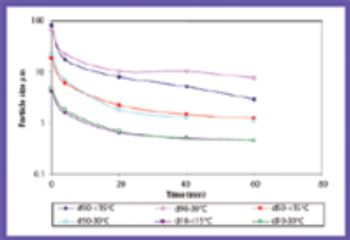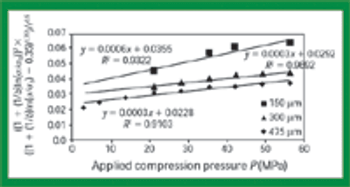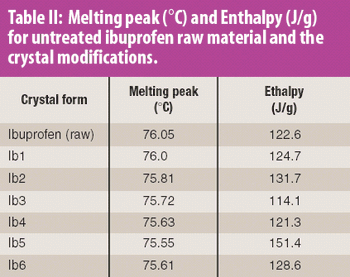
The common crystal form of ibuprofen was changed to optimize processing and manufacturing properties. Six modified crystal forms were prepared and assessed for dissolution, morphology, particle size, density, thermal characteristics, powder x-ray diffractometry, flow properties, and tabletability.