
Pfizer's Lipitor patent revoked in Germany, promotions at Charles River Labs, more.

Pfizer's Lipitor patent revoked in Germany, promotions at Charles River Labs, more.

King Pharmaceuticals, and Acura Pharmaceuticals have entered into a license, development and commercialization agreement for the United States, Canada, and Mexico, encompassing a potentially wide range of opioid analgesic products utilizing Acura?s patented Aversion (abuse-deterrent) Technology platform.

Editors' Picks of Pharmaceutical Science & Technology Innovations

Market demand for cytotoxic drugs is leading CMOs to expand their API manufacturing and formulation services.
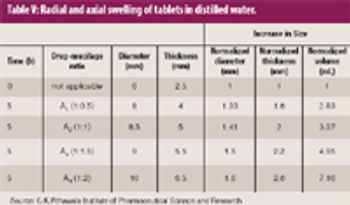
Natural gums and mucilage have been widely explored as pharmaceutical excipients. The goal of this study was to extract mucilage from the leaves of Aloe barbadensis Miller and to study its functionality as an excipient in pharmaceutical sustained-release tablet formulations.

A book illustrates the potential for nanoparticulate drug delivery, and how much about them remains to be understood.

Vaccines are needed against old and new infectious disease threats - polio and other childhood illnesses, bioterrorism and pandemic flu. They are also emerging for cancer immunotherapy and for treating addiction. While vaccines are among some of the most successful biotech products, their large-scale manufacture involves some special demands, such as maintaining a good working cell bank and gearing up for production on an 'as needed' basis.

The crystalline structure of pharmaceutical solids can sometimes be altered during processing. X-ray powder diffraction and near infrared spectroscopy can be used to determine the amorphous and crystalline content of a model substance. The two techniques' precision, accuracy, detection limit and the speed of analysis are compared.

Company Notes: Gibraltar expands facilities; Allozyne appoints new president and CEO, more.

The pharmaceutical market is undergoing a major transformation with companies moving more and more toward the generics business, according to a new report.

Company and People Notes: ImClone, BMS, and Merck form agreement; CRI Worldwide names new CEO, more.

Company and People Notes: Orexo acquires Biolipox, Avalon cofounder resigns, more...

Pfizer decided to stop investing in ?Exubera? (inhaled insulin), a product that had been considered a potential blockbuster drug.

An exclusive licence agreement has been signed to develop a new oral treatment for rheumatoid arthritis (RA), a condition that affects approximately 165 million people.

Company and People Notes: Novartis and MIT to study continuous processing, GSK appoints Andrew Witty as CEO, more.

The US Food and Drug Administration launched a new program to increase the number and variety of generic drugs available to the public, beginning in FY2008.

Company and People Notes: Thermo Fisher Scientific buys Priority Solutions International, Wyeth elects new president and CEO, more.

Transdermal delivery takes up once-forbidden compounds, reviving markets and creating formulation opportunities.
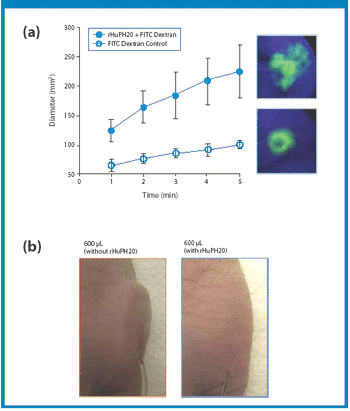
The preferred route of administration for an injected therapeutic agent is subcutaneous (SC), but SC injections are generally limited to no more than 1-2 mL in volume, representing a major challenge, especially for large protein biologics.
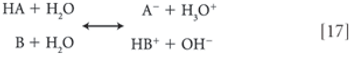
Through consideration of the ionic equilibria of acids and bases, one may readily calculate the formation constant of a salt species solely on the basis of knowledge of the pKA value of the acid and the pKB value of the base.

Orally disintegrating tablets (ODTs) continue to attract attention as an alternative to conventional oral dosage forms.
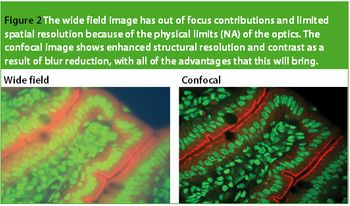
In the biological arena, new and highly useful fluorescent markers are used to stain or 'label' specific structures of interest. They have transformed the range and applicability for optical observation. These labels are excited and correspondingly emit at specific wavelengths; thus, different facets of a specimen can be 'selected' by controlling the wavelength of the delivered and captured light. For example, labels such as 4',6-diamidino-2-phenylindole (DAPI) are used to highlight the nucleus of a cell and MitoTracker Orange is used for mitochondria. Figure 1 shows an example of a multiple stained section, viewed in fluorescence. There has been an explosion in fluorescent labels for examining biological structures, in fixed and live cell preparations.

Last year, a monoclonal antibody, TGN1412, led to potentially fatal adverse effects in a small group of Phase I volunteers in London. In the wake of this incident, EMEA has drawn up new guidelines that could lead to demands for more data on novel biologics. They may have implications for both manufacturing and clinical trials of biopharmaceuticals that are considered to pose a high risk to patients.

The UK, with its high quality educational capabilities in bioscience, has an opportunity to become the global hub of bioscience training, but must act quickly to secure this position.