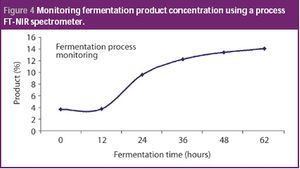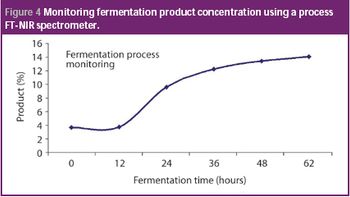
Quality by design and PAT approaches are increasingly being used for the biotech manufacturing of medicines. Complex manufacturing processes can not only be controlled using PAT principles, but optimized with respect to both product quality and economic value. This column describes how the fermentation process is often the first to benefit from this type of implementation.