Formulation and Drug Delivery
Latest News
Latest Videos

More News

Under the agreement, AstraZeneca will use Alteogen’s proprietary hyaluronidase platform technology to develop and commercialize subcutaneous formulations of multiple oncology assets in its portfolio.

Rivaroxaban, long known under the brand name Xarelto, is an anticoagulant, a classification that is among the most-prescribed medications in the United States.
Exosomes, polymeric nanoparticles, and DNA nanostructures offer many potential advantages.

The approval makes Evrysdi the first and only tablet for treating spinal muscular atrophy.
European biopharma companies are looking beyond GLP-1s.

Increasing API and formulation complexity and new delivery strategies are driving innovations in taste-masking.
Rapid market growth in biologics is helping the packaging market expand as well, but the complex nature of biologics and the desire for personalized therapies present unique hurdles to make packaging solutions cost-effective and safe for patients.

Process consistency and robustness, analytical excellence, and regulatory compliance are essential in the scale-up of biosimilars.

Winners in this year's Pharmapack Awards include both commercial products and innovations that are shaping the future of pharmaceutical packaging.

Lonza will work with Iconovo to develop spray-dried formulations for an intranasally delivered biologic using a reformulated biologic drug candidate for obesity.

The transaction, which was first announced in September 2024, expands Phillips Medisize’s inhalation drug delivery capabilities.

With the new $94 million (€90 million) funding, the company will develop its pipeline of oral macrocycle drugs, nCycles, against validated biologic targets.

With this approval, HYQVIA [Immune Globulin Infusion 10% (human) with Recombinant Human Hyaluronidase] becomes the first and only facilitated subcutaneous immunoglobulin available in Japan to treat these disorders.

Pointing to the continued circulation and evolution of COVID-19, WHO published a statement on the COVID-19 vaccine antigen composition to respond to variants of the virus.

The companies will use Orexo’s powder-based drug delivery technology to develop mucosal vaccines in an inhaled formulation.

The partners will aim to establish a platform that enables rapid development of DPI products.

Centogene NV and ROPAD consortium publish data from a landmark study identifying genetic variants that may respond to innovative cell and gene therapies.
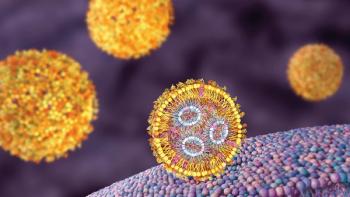
LNPs have gained solid ground as a drug delivery system for mRNA due to their success in the vaccines arena.

EXO Biologics and ExoXpert, an EXO Biologics subsidiary, have received GMP certification of a European manufacturing facility for exosomes and have successfully loaded mRNA and DNA payloads into GMP-grade exosomes for drug delivery.

With its new offering, Lonza can tailor services to support smart capsule companies with its bi-layer capsule manufacturing technology.
Datwyler has launched new coated plungers in its NeoFlex line that are suitable for large volume biologics.

The companies will collaborate to create and test circVec DNA–LNP formulations for use in potential therapeutic applications.

The partnership will use ViaNautis’ proprietary polyNaut technology platform to develop genetic medicines that can precisely target tissue types.

WHO and its partners are providing support to countries dealing with mpox outbreaks.

The report highlights international regulators’ plans for the development and availability of vaccines to prevent and drugs to treat mpox.