
Pharma companies could benefit from the lessons learned in this fall's financial crisis.

Pharma companies could benefit from the lessons learned in this fall's financial crisis.

Editors' Picks of Pharmaceutical Science & Technology Innovations

Production problems come in all shapes, sizes, and ... species.

Pharmaceutical Technology will feature video coverage of AAPS this month.

Molecules called "chaperones" facilitate correct protein folding.
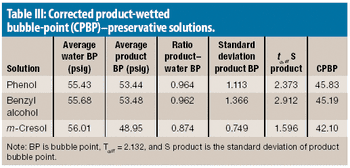
The authors explain the factors that can cause a failure in a bubble-point integrity test and what to consider when a product-specific bubble point must be defined.
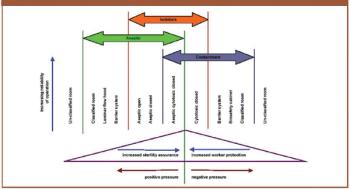
The author discusses the key issues to consider when using isolators such as containment, protection of personnel, the efficiency of biodecontamination cycles, sterility assurance levels, barriers and their integrity, and environmental impact.
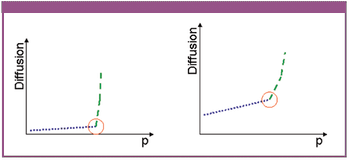
The authors describe a novel approach for the integrity testing of large sterile filter systems such as multiround housings and describe a multipoint diffusion test capable of detecting minor failures.
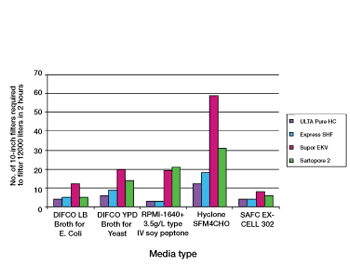
Proper selection of normal flow filters leads to increased process efficiency from early phase product development through to full-scale biopharmaceutical production.
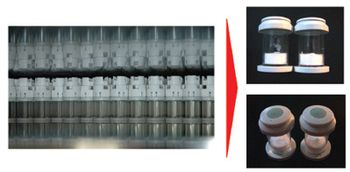
The authors present an aseptic-filling process for freeze-dried liquids using the closed-vial technology.

A comparison of conventional cleanrooms, restricted access barrier systems, and isolators, shows the benefits of using isolators in high-potency drug manufacturing.

I confess I am no financial expert. My knowledge about the current credit crisis can be summed up in six words: 'debt is bad; cash is good', which was pretty much the limit of my understanding about money before it all began.
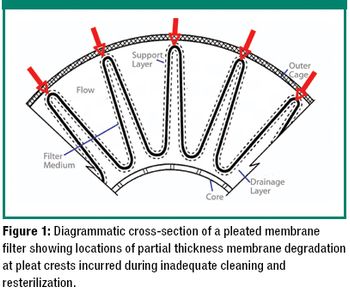
The author examines re-use of hydrophilic- or hydrophobic-membrane sterilizing-grade filters in liquid sterilizing applications.

Many industries, from aerospace to medical devices, conduct cleaning procedures.

There are many challenges upstream and downstream in manufacturing a biotech drug.
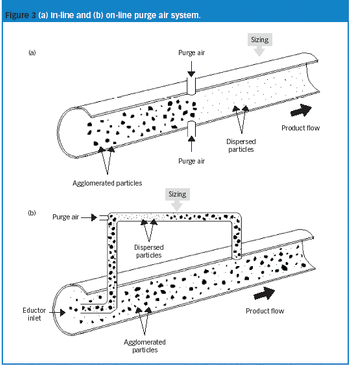
FDA advocates building quality into a product through PAT.

Many compounds fail in preclinical development because of safety-related problems, but identifying 'predictable' safety or toxicity liabilities earlier in the process could lead to improved design and selection of compounds that are more likely to be approved.

Merck plans to cut approximately 7200 positions as part of its 2008 restructuring plan, according to an Oct. 22 release focused on the company's third-quarter financial results.

Pfizer and Lilly recently resolved issues related to their marketing practices for certain products.

Also, Maxygen looks to costs, jobs; Receptor BioLogix appoints Dale R. Pfost CEO; more...

Pfizer and UCB formed a technology company named Cyclofluidic with the aim of accelerating the drug-discovery process.

The US Food and Drug Administration released a draft guidance that reviews the agency's plan to offer priority-review vouchers to companies developing new treatments for neglected tropical diseases.

Rep. John D. Dingell (D-MI), chairman of the US House of Representatives Committee on Energy and Commerce, and Rep. Bart Stupak (D-MI), chairman of the Oversight and Investigations Subcommittee, sent letters to the US Food and Drug Administration, Shaw Science Partners, and EthicAd to request information about a new FDA website.

Also, MedImmune opens Cambridge, UK, facility and makes reverse engineering pact with Omninvest; BD Medicine appoints Carol Adiletto VP of clinical and regulatory affairs; more...

Also, Millipore opens new membrane-casting manufacturing facility in Ireland; Surface Logix appoints Keith Dionne president, CEO, and a member of the board; more...