
A comprehensive book about mass transfer benefits from the author's personal touch.
Theodore H. Meltzer is principle of Capitola Consultancy.

A comprehensive book about mass transfer benefits from the author's personal touch.
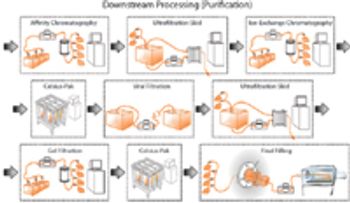
The authors discuss current and future disposable technologies and outline the validation and qualification steps that would be required for a possible disposable process stream.
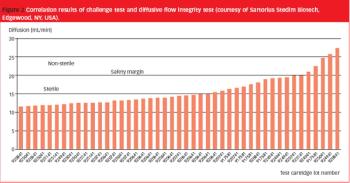
Although realized in 1983, ASTM's standrad test method for determining bacterial retention of membrane filters used for liquid filtration is still hugely beneficial to today's pharmaceutical industry.

A well-balanced guide to industrial bioseparations provides valuable information.
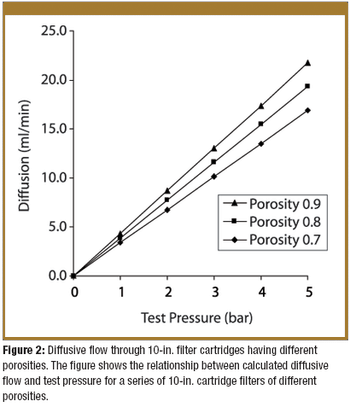
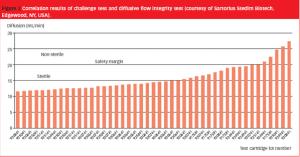
Pre-use integrity testing of sterilizing-grade filters eliminates the potential adverse effects of filter loading on the integrity-test results, allowing unambiguous correlation with the integrity-test specification established during filter-validation studies.
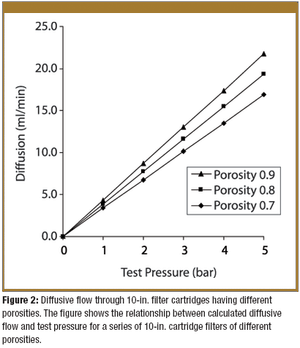
Pore-size ratings are so unrelated to actual dimensions and so subject to anomalous interpretations as to make substantial dependency upon their values an unwise choice. Moreover, the means of measuring them are questionable. The pore-size rating system at best provides a qualitative differentiation.
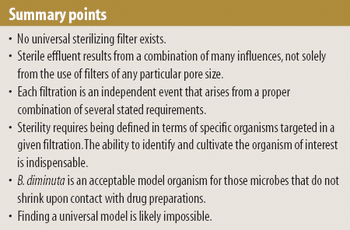
Model organisms are useful when validating sterile filtration, but successful retention of the model organism does not always guarantee that effluent is sterile. The authors explore the various factors that influence sterile filtration.

The authors encourage the investigation into whether the occurrence of grow-through and the diminution in the size of certain organisms when in contact with given liquids are the same phenomenon manifested under different circumstances.

The regulating authorities have, it seems, a preference for the application of multimembrane combinations to maximize organism retentions.

Sterilizing grade filters are widely used in the biopharmaceutical industry and were once thought of as being perfect. However, these filters have experienced rapid developments and improvements during the last decade, which have resulted in enhanced thermal and mechanical resistance. Moreover, their performance levels have been raised, which has led to significant cost savings within production processes.

Published: January 2nd 2010 | Updated:

Published: November 2nd 2008 | Updated:
Published: May 1st 2009 | Updated:
Published: May 1st 2007 | Updated:
Published: December 1st 2008 | Updated:

Published: February 1st 2006 | Updated: