
Will international biomanufacturing outsourcing become mainstream in this decade?

Will international biomanufacturing outsourcing become mainstream in this decade?

PharmTech speaks to Ray O'Connor from the National Institute for Bioprocessing Research and Training (NIBRT) for an overview of aseptic processing.

Siegfried Schmitt, a principal consultant with PAREXEL, discusses the EMA's guideline on process validation and how it compares with FDA's process validaton guidance.

Vaccine manufacturing was predicted in 2008 to be on the cusp of a golden era, but instead the industry has experienced cost pressures, high-profile liability cases and production setbacks.

Dr. Charles Kettler, director of Natoli Scientific, looks at the challenges that tablet scoring poses to tablet manufacturers.
Shortages and compounding disaster spur efforts to overhaul manufacturing oversight and stimulate industry improvements.

Tim Freeman of Freeman Technology explains how new analytical technologies have influenced quality criteria and standards for the uniformity of dosage units, and why more accurate systems are leading to greater focus on tablet scoring.

When splitting unscored tablets, the main concerns relate to API dosage control and the potential modification of time-release characteristics. PTE speaks with tableting experts Thierry Menard, Lab Manager, And Bruno Villa, President, both at Medelpharm, about how manufacturers are approaching the challenges.
Tablet splitting is a new area of focus for regulators. The FDA tells PTE more about the challenges in this area.

Room-temperature sterilization using nitrogen dioxide gas provides benefits for sterilizing the external surfaces of single-dose, parenteral drug containers.

Plastic is finding increased use in vials and syringes as concerns about glass breakage and delamination and desire for increased functionality lead pharmaceutical companies to consider alternatives.

The company issues voluntary recall of certain lots due to possible contamination with small glass particles.

As the battle against counterfeits gains momentum, PharmTech speaks to Mark Davison, CEO of Blue Sphere Health, and Craig Stobie, global life sciences sector manager at Domino Printing Sciences, about the challenges involved.

In addition to globalisation, high financial rewards and low penalties for counterfeiters are contributing to the rise in fake medicines.

To ensure the accuracy of scientific testing, protect the subjects to avoid data contamination.
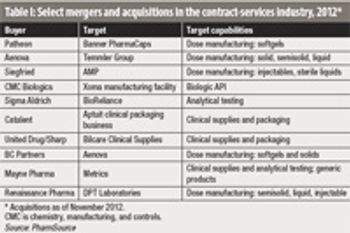
The quest to build critical mass and broaden capabilities has been a key factor in deal-making.

GlaxoSmithKline recently developed a novel technology for the formulation of modified-release tablets. The authors describe the route from development to commercialization.

Annual employment survey results show greater confidence in the pharma industry.

Can postapproval FDA filings immunize pharma companies from patent lawsuits?

A look at the year's leaders in innovation strategy, including the top bio/pharmaceutical companies and award recipients from AAPS, PhRMA, and CPhI.

Adeline Siew PharmTech speaks to Lynne Byers and Brian Johnson about Rx-360's initiatives to protect patient safety.
A change in terminology could emphasize patient protection.

Domestic companies are changing their business models in response to recent drug price cuts.
As 2012 comes to a close, Pfizer leads among Big Pharma companies for FDA approvals of new molecular entities and biologics.
Clean-in-place systems should be optimized during design and commissioning and after validation.