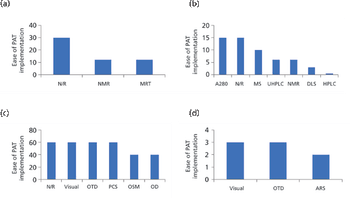
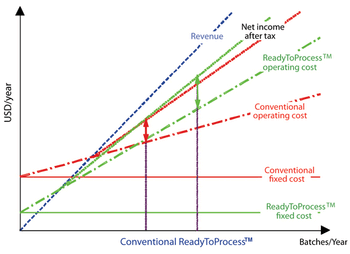
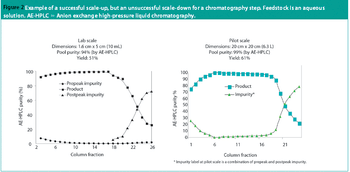
This article presents some key differences between the US and European regulation of biosimilars, including naming conventions and pharmacovigilance of biosimilars, and the impact of biosimilars on commercialization and affordability of biotherapeutics.