
Greater sophistication in 3D X-ray imaging technology raises its utility for QA/QC in manufacturing.

Greater sophistication in 3D X-ray imaging technology raises its utility for QA/QC in manufacturing.

The benefits of single-use systems are being realized for downstream unit operations, including aseptic filling.

Combination product design takes second prize in the National Collegiate Inventors and Innovators Alliance Biomedical Engineering contest.

Upcoming requirements in the US and around the world for serialization and track and trace of pharmaceuticals were a focus of the Pharmapack conference held in Philadelphia, PA earlier this week.

Model-predictive design is applied to solid-dosage processes.

Solid-dosage forms and parenteral products benefit from next-generation packaging machines.

A screening method predicts delamination in primary packaging.

PSL has developed a microsphere refiner for microsphere formulation, from small scale processes to commercial production.

Aseptic connectors provide the flexibility and robustness needed for modern parenteral manufacturing operations.

Conjugated vaccines are meeting the need for longer-lasting immune responses, but the production process is complex, and manufacturers are looking for simpler solutions.

EMA's revised guideline on biosimilars containing biotechnology-derived proteins is published for public consultation.

Facility will use mammalian cell-culture technology and be ready for operations by 2016.

Modular containment room at Belfast facility allows studies of biologics and vaccines.

Pfizer had gained the facility through its previous acquisition of King Pharmaceuticals.

The M5 Quick-Lock mounting system on Sharpe Portable Sanitary Mixers provide enhanced locking force for installation and repositioning without tools.

The Fluid Air Tablet Coating System consists of a modular air atomizing manifold, liquid-delivery skid, and Batch Architect process controls.

The Solidlab laboratory plant combines powder mixing, granulating, and coating of pharmaceutical pellets and tablets in one single machine.

The Silverson Ultramix can provide high-shear mixing in vessels with narrow openings.

Bills to regulate drug compounding and establish a national track and trace system face political and policy differences.
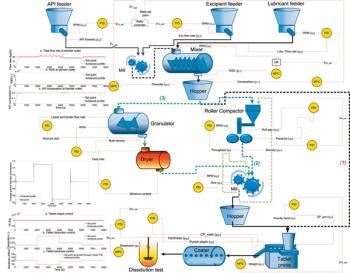
Applying model-predictive methods and a continuous process-control framework to a continuous tablet-manufacturing process.
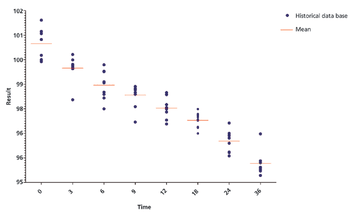
The authors discuss three methods for identification of out-of-trend (OOT) results and further compare the z-score method and the tolerance interval in OOT analysis for stability studies.
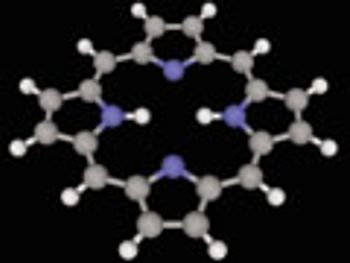
Researchers at the Scripps Research Institute advance heteorcylic chemistry trhough new reagents and reaction-tracking techniques.

A recent study of computer-created and natural proteins suggests the number of sites where small-molecule drugs can bind to proteins is limited, thereby narrowing how to mitigate side effects through drug design.

Models and modeling software gain a foothold in solid-dosage manufacturing process design.

Crushing, fracturing, and bending tests quantify hardness.