
Adoption of single-use systems and more flexible systems drive innovation.

Adoption of single-use systems and more flexible systems drive innovation.

Greater automation and the adoption of solid versus perforated pans are some recent advances.

The growing demand for targeted biopharmaceutical therapies is driving interest in modular production facilities that can be rapidly constructed while still minimizing risk and cost.

Dynamic testing and advances in shear testing provide better insight into powder physical properties and external variables that affect powder behavior.
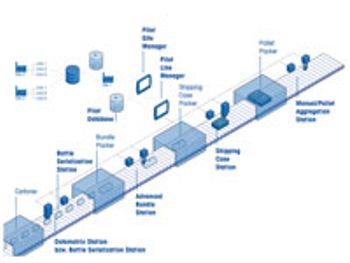
Integrated serialization systems keep pace with industry demand.

Crushing, fracturing, and bending tests quantify hardness.

Making the effort to apply new methods to pharmaceutical processing will bring benefits.

New Center for Pharmaceutical Advancement and Training increases number of experts and available tools in Sub-Saharan countries.

Drug manufacturers today are increasingly challenged to find new, effective methods for the delivery of active pharmaceutical ingredients, and many are discovering that orally disintegrating technologies can help.

New platform technologies and polymer chemistries may facilitate self-administration, longer-term delivery, and targeted delivery of parenteral drugs.

Pfizer has launched a prescription-fulfillment website for Viagra tablets in an effort to combat the online sale of counterfeited medicine.

Podcast interview with Nick Johnson, Strategic Marketing Director for Modified Release Technologies at Catalent Pharma Solutions

Applications of ZFN technology in biopharmaceutical cell-line engineering.

While there are those who want combination products to be controlled by a centralized pharmaceutical-type approval system, the majority of the medical technology industry wants to retain a decentralized device-focused approach.

The rejection by India's Supreme Court on Novartis' Glivec/Gleevec (imatinib mesylate) and other recent case law raise important issues on patent strategies for solid forms.
The impact of new delivery technologies in designing peptide therapies.

Solid-state chemistry is an important part of drug development, and public research is advancing the field.

The author suggests co-opetition as a future model for collaboration in drug development.

Lyophilization technologies for controlled nucleation.

Cocrystals are used to improve the performance of APIs that have non-ideal physiochemical properties by cocrystallizing the API with a second compound that modulates the API to provide a way to improve a drug's bioavailability, stability, and processability.

New product reviews for May 2013, featuring automation, IT, and process control systems.

Drug companies team up with INTERPOL to keep counterfeit medicines off the Internet and out of the hands of patients.

Pharmaceutical Technology spoke with INTERPHEX 2013 conference-session presenters to gain insight on trends in facility and process design.

Optimized freeze-drying cycles can offer scientific and business advantages.
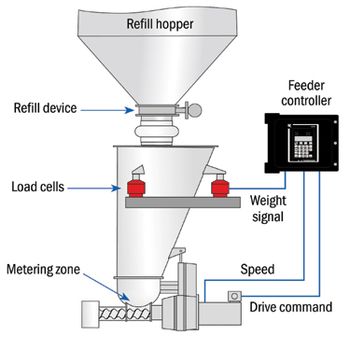
Loss-in-weight feeders provide high accuracy for batch or continuous processes.