
The Italian Medicines Agency lifted a temporary hold on the use of Novartis seasonal influenza vaccines in Italy after affirming the vaccines' safety and efficacy.

The Italian Medicines Agency lifted a temporary hold on the use of Novartis seasonal influenza vaccines in Italy after affirming the vaccines' safety and efficacy.

An operation spanning 16 African countries and conducted by the World Customs Organization (WCO) in partnership with the Institute of Research against Counterfeit Medicines (IRACM) led to the seizure of more than 82 million doses of counterfeit medicines.

A two-day workshop on the "science behind pharmaceutical stability" was held in conjunction with the Annual Meeting of American Association of Pharmaceutical Scientists (AAPS) on Oct. 21-22, 2011 in Washington, DC.

Policymakers must balance fundamental issues involving access to medicines and pricing.

A Q&A with Yves de Montcheuil, vice-president of marketing at Talend, a provider of open-source integration software.
Consider critical process parameters and strategies to optimize the manufacturing process.

Even when all is well at the facility, one must expect the worst while braving the elements.
The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on biosimilar monoclonal antibodies (mAbs).

Regulatory bureaucracy in Europe coupled with the demand for lower-priced medicines continues to hinder efforts in innovation for Alzheimer's disease.
Pharmaceutical companies and contract service providers adapt strategies and capabilities to reduce costs and accelerate drug-development timelines.

A two-day workshop on the "science behind pharmaceutical stability" was held in conjunction with the Annual Meeting of American Association of Pharmaceutical Scientists (AAPS) on Oct. 21-22, 2011 in Washington, DC.

The government of Turkey is drawing up a program in coordination with the pharmaceutical industry to create ways to make the country a regional production center for pharmaceuticals serving Europe, Central Asia, and the Middle East.

This article introduces"mean kinetic relative humidity" for evaluating the impact of humidity variability.

Overt and covert packaging technologies evolve to authenticate drugs and fight counterfeits.

The US Pharmacopeia's revised General Chapters on elemental impurity limits and testing procedures are set to take effect in December 2012.
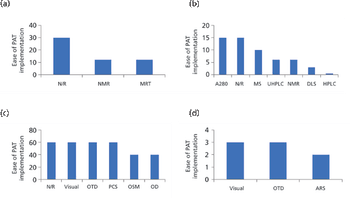
In this paper, the authors review the various analytical methods that can enable use of PAT.

New systems that combine Raman spectroscopy with automated imaging support the efficient gathering of such data, including information concerning size and shape distributions for individual components within a formulation.
This article focuses on the growing need for effective data management in the life sciences industry-especially among smaller pharmaceutical manufacturers.

Developing analytical methods and performing related testing is crucial for ensuring the quality of a pharmaceutical product.
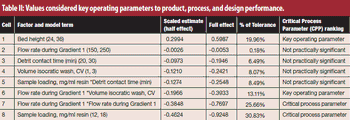
Critical process parameters (CPPs) and their associated process controls are crucial to drug development and process validation and to the evaluation of every manufacturing unit operation.

Patheon will acquire softgel-specialist company Banner Pharmacaps, based in North Carolina in the US, for $255 million

On Oct. 22, 2012, a consortium in France announced the establishment of Europe's first ever industrial manufacturing facility dedicated to the large-scale production of novel, advanced cell-based therapy medicinal products.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the October 2012 edition from UFP Technologies and Mettler Toledo.

Strategic management of intelligent devices is important in any processing field, and a new standards committee at the International Society of Automation aims to provide direction so that manufacturers can better utilize the devices' capabilities.

Consider these tools and strategies for optimizing the manufacturing process.