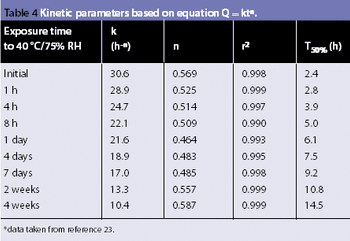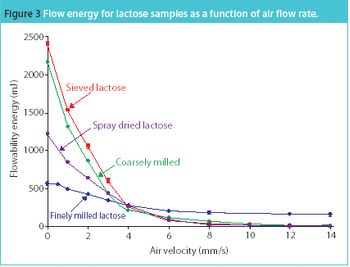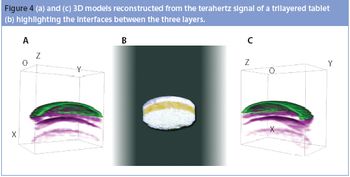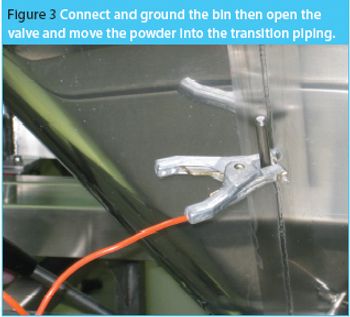
The same phenomena that create lightning and thunderstorms are around us every day, producing incredibly high voltages, which cause sparks and shocks. Static electricity is a mighty force. Each year excessive electrical charge build cause explosions in the grain industry.1 Look around any flammable storage area and you will see both grounding bars on the wall and cables, from the grounding bars connected to the drums of solvents. Take any material safety data sheet (MSDS) for a powder and look in section V; it highlights that any dry powder has the potential to attract and store a charge.