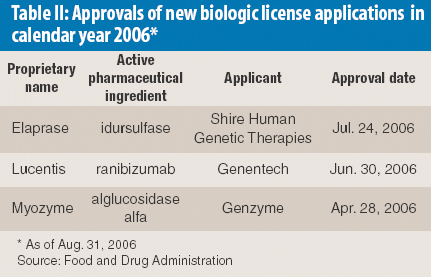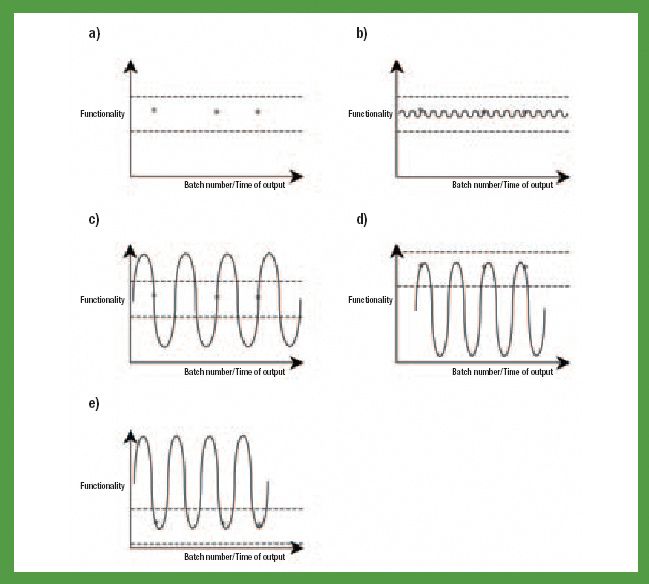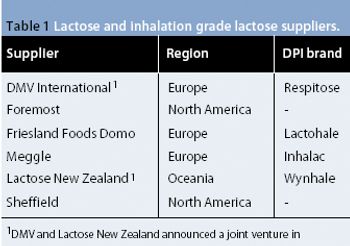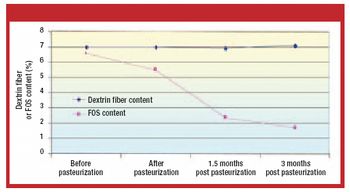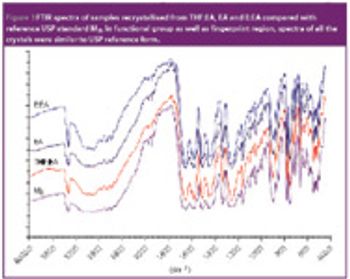
Mefenamic acid has variable bioavailability and tabletting issues because of its hydrophobic nature and poor material characteristics. Recrystallization of mefenamic acid was performed from three different solvent–solvent mixtures under differing conditions. The crystals obtained were screened for the existence of new crystal properties or polymorphic forms, then characterized further.