
Recent technology improvements have made acrylics the preferred system for the aqueous enteric coating of tablets.

The process of extrusion/spheronization used to produce spherical granules frequently relies on formulations containing microcrystalline cellulose (MCC). This excipient can hold water, even when pressure is applied, and form "pastes" that have suitable rheological properties, which allow both extrusion and subsequent spheronization to produce uniform spherical granules. This article describes a new approach to providing paste systems with appropriate characteristics. This can be achieved by incorporating glyceryl monostearate (GMS) into the formulation. It was found that the inclusion of GMS in formulations provides a useful alternative to MCC as an effective excipient to aid the preparation of spherical granules, allowing the incorporation of drug loads as high as 90%.

Recent technology improvements have made acrylics the preferred system for the aqueous enteric coating of tablets.

The author describes a separation method for two active ingredients in the contraceptive pill with liquid chromatography UV detection.

The author suggests that an excipient's functionality can only be determined in the context of a specific formulation and manufacturing process.

Dry powder inhalers are a well-accepted dosage form for pulmonary drug delivery and a wide variety are either currently available or in development. This article examines a premetered, capsule-based multidose inhaler for which different qualities of a-lactose monohydrate were screened.
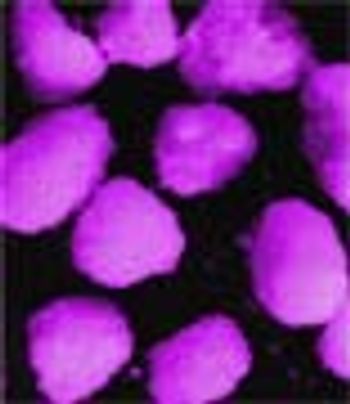
Previous articles have presented a general review of the different types of spheres that can be obtained with a rotary fluidized bed process.1,2 This two-part study focusses on lipid spheres that can be prepared using hydrogenated castor oil, as well as examining the feasibility of the process and the main characteristics of the spheres obtained.

Erythritol is a bulk sweetener polyol that is suitable for a variety of reduced-calorie and sugar-free foods. It has been part of the human diet for thousands of years because of its presence in foods such as fruit, mushrooms and fermentation-derived products including wine, soy sauce and cheese. This article investigates the properties of erythritol and describes how it can be used as a pharmaceutical excipient.

The tabletting properties of a new coprocessed excipient for direct compression were compared with a physical mixture of its components (separately and with drugs) and the individual constituents. The compaction properties were also investigated. Results indicated that the new excipient has excellent flow properties and demonstrates enhanced compressibility.
Medicines and excipients are inseparable, with few exceptions - one cannot exist without the other. The Pharmaceutical Quality Group and other international bodies have developed good manufacturing practice (GMP) standards and guidelines to facilitate the effective supply of excipients. This article discusses the definition and significance of excipients, and highlights the importance of implementing the correct excipient manufacturing controls and standards.

The aim of this work was to investigate the compactibility, compressibility and drug release behaviour of different fractions of a commercially available ethyl cellulose.

Dry powder inhalers (DPIs) contain a powder which, when required, is discharged and inhaled. The therapeutic drug is manufactured in powder form as small particles a few micrometres in diameter. In many DPIs, the drug is mixed with much larger sugar crystals, such as lactose, and the smaller drug particles attach to these excipient particles, improving entrainment of the drug upon inhalation. This article examines how the application and combination of versatile processes such as milling, micronizing, sieving and air classification can be used to manufacture dedicated lactose products for practically every possible combination of active and excipient blend in DPIs.

Using melt extrusion to prepare glass solutions of poorly water-soluble drugs with hydrophilic excipients offers an exciting and advantageous alternative to existing formulation methods such as spray-drying and co-melting. Investigating potential methods to increase water solubility begins early in drug development. Techniques described in this paper show how only a small quantity of drug can be used to determine its suitability for melt extrusion, allowing the method to be considered at the same time as salt screening and particle size reduction work, and could speed up the formulation process.

USP ensures the microbiological quality of products by way of the development, proposal, and acceptance of general information chapters.

Appropriate sampling procedures, proper preparation, and correct instrumental parameters may yield differing, but correct, results.

Recent technology improvements have made acrylics the preferred system for the aqueous enteric coating of tablets