SAFC is delivering on its plan for double-digit annual growth by increasing its businesses through organic growth and targeted technology acquisitions.

SAFC is delivering on its plan for double-digit annual growth by increasing its businesses through organic growth and targeted technology acquisitions.

The selection of an appropriate salt form for a potential drug candidate is an opportunity to modulate its characteristics to improve bioavailability, stability, manufacturability, and patient compliance.
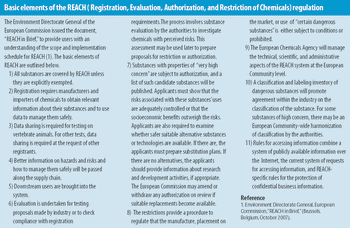
The European Union's REACH initiative has the potential to affect the flow of chemicals into the pharmaceutical suppy chain.

Show blasts off this month in Philadelphia with more suppliers, new trends, and real-world solutions.
A news roundup for March 2008.

Brief pharmaceutical news items for March 2008.

Also, Genmab will acquire PDL Pharma's Minnesota manufacturing facility, DSM Pharmaceuticals appointed Hans Engels president and business unit director, more...

The US Patent and Trademark Office rejected the patentability of claims of a patent by Genentech that related to certain methods used to make antibodies and antibody fragments by recombinant DNA.

APP Pharmaceuticals will boost manufacturing of therapeutic multidose vials of heparin.

Also, Biocon will acquire 70% of AxiCorp, ARIAD Pharmaceuticals promoted Richard W. Pascoe to the new position of COO, more...

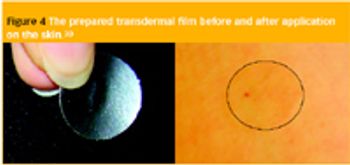
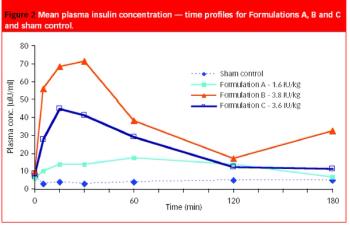
To ensure an effective treatment, a patient often must take equal doses of an active pharmaceutical ingredient (API) at regular intervals.

Chesapeake to relocate and expand, AVI BioPharma appoints CEO, More...

Contract manufacturers of active pharmaceutical ingredients and intermediates unveil expansion plans and strategies at this year's Informex.

A recent business outlook survey conducted by the Synthetic Organic Chemical Manufacturers Association reveals a generally favorable view of current and future business conditions for contract manufacturing of active pharmaceutical ingredients and intermediates.

Amira and GSK Form Agreement, Xenome Appoints Ian Nisbet CEO, More...

Contract manufacturers expand capabilities in aseptic processing, clinical-trial materials supply, and cytotoxic manufacturing.

The rise in overseas manufacturing undermines FDA oversight of drug quality.

PharmTech's polls feature user feedback on issues facing the pharmaceutical industry.

Brief pharmaceutical news items for February 2008.

A news roundup for February 2008.

Can an overload of patent applications lead to the US' demise as a scientific leader?

A wave of pharmaceutical expansions is expected in Europe this year, surprisingly by Indian companies.

Codexis Opens Budapest Lab, Allos Names Bruce K. Bennett VP of Manufacturing, More...