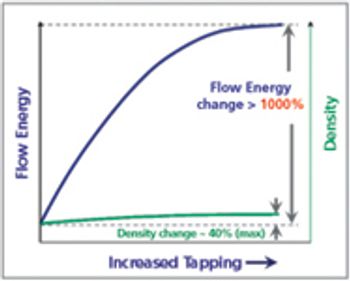
When splitting unscored tablets, the main concerns relate to API dosage control and the potential modification of time-release characteristics. PTE speaks with tableting experts Thierry Menard, Lab Manager, And Bruno Villa, President, both at Medelpharm, about how manufacturers are approaching the challenges.