
Efforts to tackle challenges in tablet manufacture are shaped by quality by design (QbD) and continuous manufacturing.
Tim Freeman is Managing Director of powder characterization company Freeman Technology for whom he has worked since the late 1990s. He was instrumental in the original design and continuing development of the FT4 Powder Rheometer® and through his work with various professional bodies, and involvement in industry initiatives, is an established contributor to wider developments in powder processing.Tim has a degree in Mechatronics from the University of Sussex in the UK. He is a mentor on a number of project groups for the Engineering Research Center for Structured Organic Particulate Systems in the US and a frequent contributor to industry conferences in the area of powder characterisation and processing. A past Chair of the American Association of Pharmaceutical Scientists (AAPS) Process Analytical Technology Focus Group Tim is a member of the Editorial Advisory Board of Pharmaceutical Technology and features on the Industry Expert Panel in European Pharmaceutical Review magazine. Tim is also a committee member of the Particle Technology Special Interest Group at the Institute of Chemical Engineers, Vice-Chair of the D18.24 sub-committee on the Characterization and Handling of Powders and Bulk Solids at ASTM and a member of the United States Pharmacopeial (USP) General Chapters Physical Analysis Expert Committee (GC-PA EC).

Efforts to tackle challenges in tablet manufacture are shaped by quality by design (QbD) and continuous manufacturing.
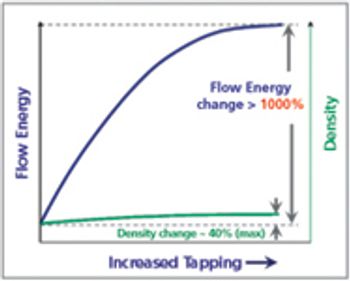
This article considers the different conditions to which the powder is subjected in the tableting process, and discusses which powder properties should be measured to accurately reflect likely powder behavior in the process.

Continuous manufacturing is increasingly noted as an important long-term objective for the pharmaceutical industry. PTE talks with Tim Freeman, Director of Operations at Freeman Technology, about some of the central issues involved in this transition, as well as the supporting role of relevant analytical technology.
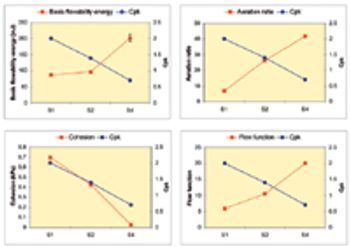
The author explains how to gain an understanding of the relationships between powder characteristics and process performance to match filling-machine geometry to the demands of specific formulations.

The unit selected for wet granulation, whether it be a high shear mixer or a fluidised bed, has a marked impact on granule properties.

How to adapt a real time release approach to powder processing during drug-product manufacturing.

How effective powder characterization can lead to better powder understanding and control.

The needs of the pharmaceutical industry for powder testing technologies are changing, primarily for two reasons. The first is that global economics, and the economics and competitiveness of the pharmaceutical industry itself, are driving manufacturers towards achieving greater efficiency.

The needs of the pharmaceutical industry for powder testing technologies are changing, primarily for two reasons.
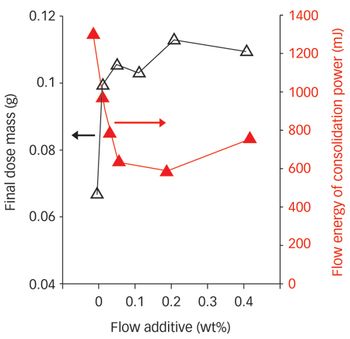
Enshrined in the concept of Quality by Design is the premise that optimized pharmaceutical manufacturing requires detailed understanding of products and processes. With this in mind, many benefits can be achieved by combining modern powder characterization techniques with real processing experience.

The current trend within the pharmaceutical industry toward more efficient development, manufacturing, and specification is fueling demand for analytical tools that provide highly relevant information. Effective powder characterization has a valuable role to play.
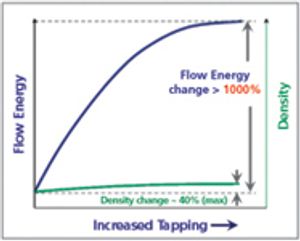
Published: March 1st 2012 | Updated:

Published: November 1st 2011 | Updated:
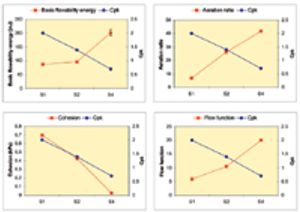
Published: April 1st 2011 | Updated:

Published: March 7th 2011 | Updated:

Published: February 2nd 2011 | Updated:

Published: June 1st 2010 | Updated: