
The pharmaceutical majors target biologics and emerging markets in their manufacturing expansion activities and plans.

The pharmaceutical majors target biologics and emerging markets in their manufacturing expansion activities and plans.
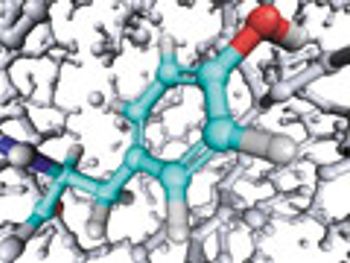
Stapled peptides offer promise to enable cell permeability, binding to therapeutic targets, and modulation of biological pathways.
Extensive comparability testing is required to ensure that biosimilars have comparable profiles to their reference products.
Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

View companies' outsourcing profiles from PharmTech's 2013 Outsourcing Resources Fast Locator Index in the following categories.

Successful product development and technology transfer between a CMO and the sponsor company requires a multifaceted collaborative approach. The author, Marga Vines from Grifols International, analyzes a project for an injectable drug for which a new drug application was submitted to FDA.

The investment activity of CDMOs and CMOs reveals the broader business and technical trends of the pharmaceutical industry.
External manufacturing plays a crucial role in pharmaceutical companies’ supply strategy. The author examines market trends for the captive and merchant global market for active pharmaceutical ingredients and intermediates.
Increased pharmaceutical trade creates new challenges for regulatory oversight.

A new method of vaccine design, called the Multiple Antigen Presentation System (MAPS), may result in vaccines that bring together the benefits of whole-cell and acellular or defined subunit vaccination.

Australian team develops method for making ultrafine particles for more efficient drug delivery.

Susan Schniepp, vice-president of quality and regulatory affairs at Allergy Laboratories and co-chair of the program planning committee for the 2013 PDA/FDA Joint Regulatory Conference, discusses quality systems and related considerations in parenteral drug manufacturing.

FDA conducted a general inspection of operations and quality systems of Albany Molecular Research Inc (AMRI) in Burlington, Mass.

Continuous countercurrent tangential chromatography (CCTC) is a column-free process that provides a scalable, disposable, and cost-saving alternative to column chromatography.

New cartridges designed specifically for use on high-speed filling lines increase efficiency.

Presenters at a pharmaceutical extrusion seminar discussed formulating drugs produced using hot-melt extrusion.

Containers, components, and quality control equipment help safeguard product quality and patient safety.

The authors describe how a combination of imaging techniques can be used to provide topographic and chemical information to characterize a sample.

Vetter has ready-to-submit documentation for this service in Common Technical Document (CTD) formats for the US, Europe and Japan.

The new ink takes less a second to dry and is four times more fade resistant than inks that are typically used in retail packaging.

The US and EU move forward with measures to fortify the pharmaceutical supply chain.

Report outlines recommended practices for control and evaluation of operations.

Novartis and Biological E, a biopharmaceutical company based in India, have entered into an agreement that aims to deliver affordable and accessible vaccines for typhoid and paratyphoid A fevers to developing countries and thereby address the unmet medical need in endemic regions.

Compounding, tracking legislation moves forward

Risk management guides decisions in facility design and operation for highly potent drugs.