
Analytical methods are being used to troubleshoot tablet-sticking problems and to develop screening methods and predictive models to more quickly find solutions.

Analytical methods are being used to troubleshoot tablet-sticking problems and to develop screening methods and predictive models to more quickly find solutions.
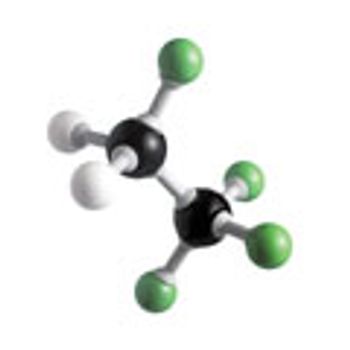
Fluorinated molecules play an important role as pharmaceutical compounds. Recent advances seek to overcome the challenges of selective and late-stage insertion of fluorine into small molecules.

3M introduces a rugged comfort respirator for harsh working environments.

An industry leader since 1933, Catalent develops and manufactures over 80% of the world's Rx softgel products with 200+ products on the market in 80+ countries.

Rockwell Automation releases a new guide to functional safety for process applications.

The collaboration with Pfizer aims to develop next-generation, continuous manufacturing for solid-dosage pharmaceuticals.

Use of a continuous-flow reaction made it possible to scale up a highly exothermic reaction for the production of a key Suzuki−Miyaura coupling reagent.

Measuring headspace gas composition and pressure allows sterile product manufacturers to simultaneously monitor important quality parameters rapidly and nondestructively.

GE Healthcare Life Sciences announced that it will build a KUBio modular biopharmaceutical factory in China for JHL Biotech.

Capsugel?s Dosage Form Solutions business unit will expand facilities in France, the UK, and the US.

Presenters at the 8th Annual Forum on Manufacturing Execution Systems (MES) discuss how a vision and common terminology help companies implement MES globally.
Featured products from Pharmaceutical Technology's monthly newsletter, Equipment & Processing Report

Helium integrity testing offers advantages over pressure-decay testing for biopharmaceutical single-use vessels.
New weapons in the anticounterfeiting arsenal include authentication and labeling technologies.

The Biomanufacturing Research Program (BioMAN) at Massachusetts Institute of Technology (MIT) has received $10.4 Million from the Defense Advanced Research Projects Agency (DARPA) to develop new technologies and manufacturing platforms that will provide an emergency supply of medicines for front-line military medics.

FDA Issues Draft Guidance on Patient Counseling Info for Labeling

FDA updates guidance to reflect advances in technology.

Foam granulation is much easier to control under mechanical dispersion conditions, which is where most industrial processes operate.

FDA specifies the DUNS UFI system for registration of domestic and foreign drug establishments.

FDA revises boxed warnings for extended-release and long-acting opioid analgesics and postmarket study requirements.

Regulatory Authorities Discuss Elemental Impurities Standards

Mylan completes expansion of its transdermal patch facility in Vermont.

FDA inspections reveal possible inaccurate sterility testing.

Following passage in the California Assembly, the California State Senate passes legislation to specify requirements for dispensing biosimilars.

Merck & Co gets FDA approval to manufacture bulk varicella at its in Durham, North Carolina for use in chickenpox and shingles vaccines.