
I Holland has introduced the next generation to its range of MF polishers, the MF40 automated punch, and die polishing machine.

I Holland has introduced the next generation to its range of MF polishers, the MF40 automated punch, and die polishing machine.
The adoption of quality by design in small-molecule drug development and manufacturing continues to evolve as the industry seeks ways to augment process understanding for APIs

The advantages of using an automated powder dispensing system in a ventilated balance enclosure for efficient handling and effective containment of potent compounds are discussed.

Industry experts discuss the requirements and challenges involved in getting a biosimilar product from bench to launch.

Omega Design Corporation offers the LabelSync 450 Vision Module, designed to capture and sync a bottle?s unique serialized label with its individual line code.

The Bosspak VTC 100 electronic tablet and capsule counter from Romaco?s is designed to fill pharmaceutical solids or food supplements into bottles at high speed.

Restricted access barrier systems (RABS) maximize product control but minimize operator interaction in aseptic manufacturing.

CentuRecon enables dry formulations of therapeutic proteins to quickly be prepared for injection at high concentration.

John Yin, an applications specialist with Freeman Technology, discusses the importance of powder-characterization techniques for optimizing pharmaceutical product development and manufacturing processes.
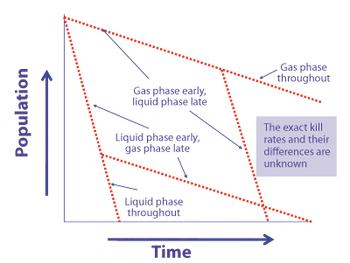
Vaporous hydrogen peroxide, used for sterilization and decontamination, is highly potent but presents implementation challenges.
Industry experts share their views on the outsourcing model and the current and future direction of contract chemical API manufacturing.
Commercial-scale amide formation and an improved process route for a tetracycline derivative are some recent developments in API synthesis.

A recent FDA guidance places greater attention on cocrystals as a tool in solid-dosage formulation.
The authors describe the background and applications of fully formulated aqueous shellac for film-coating systems.
Case studies on the manufacture of a bluk powder and the development of a tablet show the application of QbD principles.

A new standard from the International Society of Automation addresses information technology security solutions for manufacturing applications.

Cubist issued a voluntary recall of four lots of vials due to the presence of particulate matter.

Conventional cell-imaging systems that provide high quality data can be expensive and complex to use. New systems from Biotek Instruments are designed to overcome these issues.

Installation of a quaternary chiral center with high enantioselectivity using memory of chirality enabled the six-step synthesis of the desired active compound.

Direct tablet compression is simpler than wet or dry granulation, but obtaining the right flow properties to ensure good compaction and uniform drug distribution can be a challenge. Porous silica gel, when used as a glidant, can address these issues.

Extensive adoption of single-use technologies and unidirectional flow reduces cross-contamination risk in the company?s new biomanufacturing facility.

Dr. Reddy's initiates voluntary recall of ranitidine due to microbial contamination.

Parameter Stability Storage adds validated space in anticipation of pending contracts.

Experts, including researchers at I Holland, Natoli, and Rutgers University’s Engineering Research Center for Structured Organic Particulate Systems, are seeking a greater understanding of the fundamental causes of tablet sticking and are developing predictive models to more quickly find solutions to specific sticking problems.

RABS is a flexible barrier system that maximizes product control but minimizes operator interaction when best practices are followed.