
Technology can solve enterprise-level problems.

Technology can solve enterprise-level problems.

Editors' Picks of Pharmaceutical Science & Technology Innovations

With pressure to cut costs, shorten the pipeline life cycle and maximize return on investment, pharmaceutical manufacturers need tools that help them improve enterprise-wide communications, reach critical decisions faster and produce timely, accurate reports on how compounds are progressing.

Also, Roche's Avastin trial does not meet endpoint; Innate Pharma names regulatory VP; more...

Also: Sanofi-aventis acquires BiPar Sciences; FDA issues Warning Letter to Chinese heparin manufacturer; Halo Pharmaceutical appoints chief scientific officer; more...

Also, Sanofi-aventis acquires Medley and Laboratorios Kendrick; Eli Lilly's Cook to retire from board; more...

Also, Genzyme and Bayer HealthCare form agreement; FDA releases draft guidances; TransMolecular appointed Robert Radie president and CEO
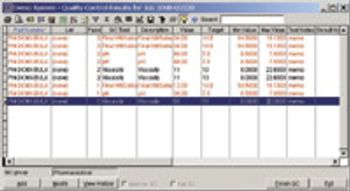
Editors' Picks of Pharmaceutical Science & Technology Innovations

Also, SOCMA changes name; two FDA approvals; Biogen Idec names chief operating officer; more...

Obama's cost-containment and science-innovation initiatives need to overlap.

The financial and economic downturn is likey to have long-term implications for outsourcing.

The role of automation suppliers is transforming to meet the pharma industry's demand for change.

Also, Hospira to reduce workforce; WuXi AppTech makes senior appointments; more...

Also, Genzyme receives warning letter; Mesa Laboratories appoints John J. Sullivan CEO and a member of the board of directors; more...

Also, Penn Pharma to expand; stem cell research funding ban lifted; Bristol-Myers Squibb made senior appointments; more...

Also, Schering-Plough's vaccine unit, Nobilon, formed an agreement with the World Health Organization; Ore Pharmaceuticals named president and CEO; more...

Industry has changed, but its basic tenets have not. INTERPHEX's RJ Palermo discusses a 7-step process to keep pharma moving forward.
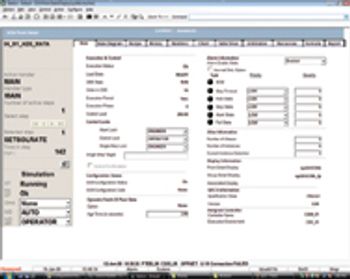
Editors' Picks of Pharmaceutical Science & Technology Innovations

Contract manufacturers of APIs and intermediates report gains, but express caution.

Also, GPC Biotech AG and Agennix to merge; BASi appoints COO of scientific services; more...

The US Food and Drug Administration posted the Prescription Drug User Fee Act (PDUFA) IV Drug Safety Five-Year Plan on its website.

Also, Sandoz received approval for its third biosimilar from the EU, WuXi PharmaTech's CFO Benson Tsang to leave at month's end; more...

To implement QbD and reduce business risks, teams should begin QbD collaboration early during process development.

Congressman John D. Dingell (D-MI) introduced HR 759, known as the Food and Drug Globalization Act of 2009, which would amend the Food, Drug, and Cosmetic Act to address food, drug, and device safety, including registration of producers of drugs and applicable fees, documentation for admissibility of drug imports, country of origin labeling, and the inspection of producers of drugs and active pharmaceutical ingredients (API).

Also, PPD to acquire AbC.R.O.; Bilcare Global Clinical Supplies named Tony Moult general manager of Bilcare GCS Europe; more...