
Also, UCB will divest certain business in emerging markets to GSK; the KineMatik Group named Michael G. Jarjour president and CEO; more...

Also, UCB will divest certain business in emerging markets to GSK; the KineMatik Group named Michael G. Jarjour president and CEO; more...

Also, Elan explores strategic alternatives; NanoGuardian appoints John D. Glover to lead its Security Advisory Board; more...

Last week, the US Food and Drug Administration issued a draft guidance for industry, Standards for Securing the Drug Supply Chain–Standardized Numerical Identification for Prescription Drug Packages, and launched a pilot program to help protect the pharmaceutical supply chain.

An integrated information technology environment can help pharmaceutical companies find new processes and approaches to improve manufacturing efficiency, productivity, and quality.

Also, Bristol-Myers Squibb forms collaboration with ZymoGenetics, Hana Biosciences appoints VP of regulatory affairs, more...

Also, Novozymes Biologicals settles pollution case with the US Department of Justice; EntreMed restructures management team; more...
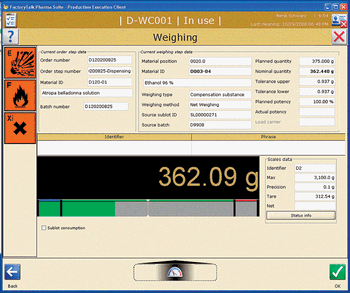
Editors' Picks of Pharmaceutical Science & Technology Innovations

The incoming administration has renewed hope for stem cells, but less adequate copycats may follow.
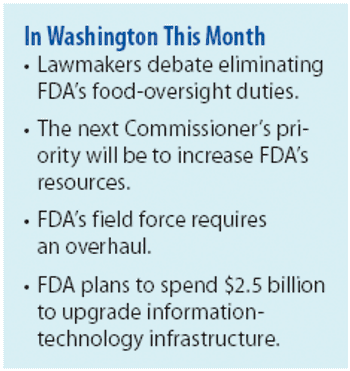
Will more resources and new leadership fix FDA, or is a major overhaul in order?

Instead of hampering progress, cost pressures might inspire Big Pharma to get creative.

Also, Crucell and DSM announce deals with GSK, Talecris, and CSL; Nobel Prize winner Luc Montagnier joins Viral Genetics; more...

Also, BASF opens lab in Mumbai; Evotek president and CEO to resign; more...

A surprising amount of the discovery and development life cycle is still based on manual and disconnected process steps.

Also, NicOx and DSM make manufacture and supply pact for naproxcinod drug substance; NovaRx appointed Norrie Russell president and COO; more...

Also, AstraZeneca announces changes to its supply chain operations; Christian Velmer appointed head of Wyeth Canada; More...

The past year saw major acquisitions attempted, completed, rejected, and stalled.

Readers provide insight into the best companies to work for as well as the ups and downs of their jobs.

China's quality approach to domestic versus exported products seems to be a lose-lose situation.

Results from Pharmaceutical Technology's Annual Employment Survey.
Pharmaceutical Technology is pleased to recognize the winners of its Innovations in Pharma Science Awards.

Also, Johnson & Johnson will acquire Omrix Biopharmaceuticals for $438 million; Charles River Laboratories promoted Foster Jordan to corporate senior VP of endotoxin and microbial detection products; more...

Also, BASi opens European office in Warwickshire, UK; Acusphere's Howard Bernstein Resigns; More...

Also, Patheon opens new European headquarters, Cynvec appoints Frank D. Stonebanks president and CEO, more...

Also, Sartorius Stedim Biotech GmbH to acquire Wave Biotech; AstraZeneca's John Patterson to retire; more...

Data capture needs to be fast and reliable...so which automatic identification technology is best?