
Biotech firms must first close the gaps between science and biology on the path toward QbD.
Company and People Notes: Nabi and GSK Form Pact; Cardinal Health Names CEO; More...

Biotech firms must first close the gaps between science and biology on the path toward QbD.

Individuals and companies at the top seem to have no problem short-circuiting their success.

Researchers working on extending the shelf life of antibody drugs may find help in a computer model developed by a research team at MIT (Cambridge, MA).

Also, Isogen completes sterile facility; Dishman appoints head of generic API business; FDA releases final rule on authorized generic drugs and NDA submissions; more...

Also, Gilead and Tibotec form agreement; FDA approves 2009-2010 flu vaccine; Catalent appoints VP in packaging unit; more...

Also, Lonza and Medarex sign agreement; Covance appoints VP and chief scientific officer of global analytical services; more...

Also, Lundbeck acquired LifeHealth; FDA is seeking a director of its new tobacco regulation branch; Charles River Labs announces personnel changes; more...

Also, Wyeth and Catalyst sign agreement; FDA seeks public opinion about tobacco regulation; Catalent appoints VP of quality and regulatory affairs; more...

REMS to improve the safe use of opioids may lead to controls on other high-risk medicines.

The regulators are doing it. But industry's fear of sharing information may leave them behind.
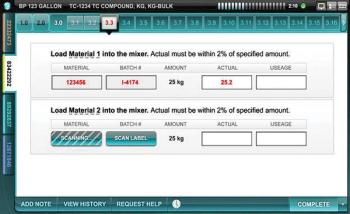
Editors' Picks of Pharmaceutical Science & Technology Innovations

Is it good policy to pay for bad behavior?

Social media is big news. From Facebook to Twitter, individuals are embracing the opportunity to participate in online publishing at an unprecedented rate. Communities of users interact globally, minutetominute within huge databases of content that they themselves create.

Good information management is essential to help pharmaceutical companies efficiently translate large amounts of data into drug market applications, achieve fast time to market and reduce costs and in Europe, many companies and regulatory agencies are actively assessing the potential of the EMEA's XML-based Product Information Management initiative.

Also, Adimab forms deals with Merck and Roche; Manhattan Pharmaceuticals' CEO and president steps down; more...

Also, FDA debars clinical investigators; Jubilant Organosys forms deal with Endo Pharmaceuticals; AMRI makes changes to its India management team; more...

Also, TorreyPines Therapeutics to liquidate assets and dissolve company; EU's competition services to examine Pfizer/Wyeth merger; Akorn appoints Raj Rai interim CEO; more...

The US Government Accountability Office recommended that the US Food and Drug Administration draft a plan, including milestones, for developing its Sentinel system and ensuring the privacy and security of patients' healthcare data.

Also, WACKER expands Iowa facility; EMEA releases a Q&A document for PIPs; Metrics consolidates quality operations; more...

A book guides readers through the regulatory requirements for computerized quality systems.

As regulators work to curb counterfeiting, industry finds benefits to gaining granular data about the supply chain. This article contains bonus online-exclusive material.

Misleading the public about their investments-be it money or medicine-is unacceptable.

It can take a lot of work to make sure nothing happens.

Also, Johnson & Johnson acquires Cougar Biotechnology; NIH launches program for rare and neglected diseases; PPD restructures leadership positions; more...

Also, Oxford BioTherapeutics forms drug development pact with GSK; Avila Therapeutics names CEO; more...