
Also, Oxford BioTherapeutics forms drug development pact with GSK; Avila Therapeutics names CEO; more...

Also, Oxford BioTherapeutics forms drug development pact with GSK; Avila Therapeutics names CEO; more...

Also, Takeda to consolidate operations in Ireland; FDA to redesign website; Archemix names president and CEO; more...

Also, Oxford BioMedica forms collaboration with sanofi-aventis; FDA requires labeling changes for botulinum toxin producs; C. Richter King joins IAVI; more...

Strong growth in biopharmaceuticals bodes well for contract manufacturing, but the perils and the promises of pipelines remain.

The president of BIO proposes the ingredients needed for industry growth.
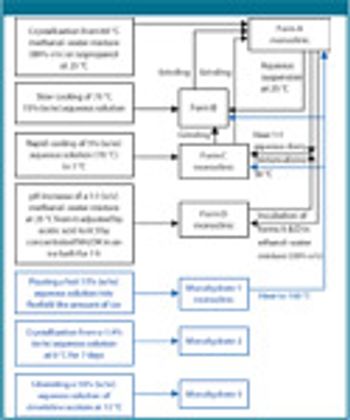
The authors describe the importance of a rapid and an abbreviated screening strategy by initial solvent screening in 20-mL scintillation vials.

The Japanese government is eager to jumpstart its generic-drug market, but changes must come first.
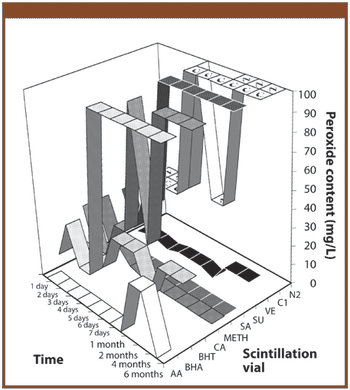
The authors investigate whether the addition of an antioxidant could be used to stabilize the solvent ethyl lactate by preventing the formation of peroxides
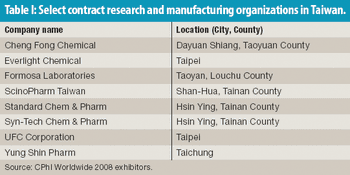
Taking a cue from its electronics industry, Taiwan is seeking to put its biotechnology and pharmaceutical industries on the map. An interactive map shows pharmaceutical activity in Taiwan.

Manufacturers of therapeutic monoclonal antibodies consider new paradigms in purification technologies.

The organizations' presidents discuss market exclusivity, approval processes, and pending legislation.

Technology can solve enterprise-level problems.
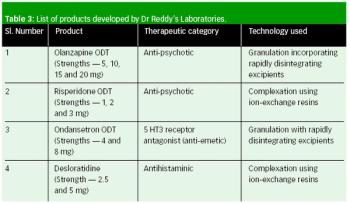
Orally disintegrating tablets offer numerous advantages compared with traditional tablets and capsules, and can be an effective solution for developing line extensions of currently marketed therapies.

Also, Roche's Avastin trial does not meet endpoint; Innate Pharma names regulatory VP; more...

Also: Sanofi-aventis acquires BiPar Sciences; FDA issues Warning Letter to Chinese heparin manufacturer; Halo Pharmaceutical appoints chief scientific officer; more...

The United States Pharmacopeial (USP) Convention signed a memorandum of understanding (MOU) with the Federal Service on Surveillance in Healthcare and Social Development of the Russian Federation (Roszdravnadzor) last week in Moscow.

Also, Sanofi-aventis acquires Medley and Laboratorios Kendrick; Eli Lilly's Cook to retire from board; more...

Also, Genzyme and Bayer HealthCare form agreement; FDA releases draft guidances; TransMolecular appointed Robert Radie president and CEO

The financial crisis is adding further injury to already weakened prospects for the pharmaceutical industry.

Follow-on biologics or biosimilars offer a niche growth market for the pharmaceutical industry. As the process for establishing a regulatory pathway for biosimilars is debated, companies, including Big Pharma, are positioning themselves to gain this piece of the pharmaceutical pie.

The US Senate introduced a bipartisan bill (S. 726) on Mar. 26, 2009, which establishes a regulatory pathway for approval of biosimilars.

Also, SOCMA changes name; two FDA approvals; Biogen Idec names chief operating officer; more...

Obama's cost-containment and science-innovation initiatives need to overlap.

A review of recent product innovations, policy developments, and growth prospects in the excipients market.

The financial and economic downturn is likey to have long-term implications for outsourcing.