
Also, Lonza and Medarex sign agreement; Covance appoints VP and chief scientific officer of global analytical services; more...

Also, Lonza and Medarex sign agreement; Covance appoints VP and chief scientific officer of global analytical services; more...

The Society of Chemical Manufacturers and Affiliates (SOCMA) this week stated its support to the US Senate for approving legislation that would extend existing chemical security standards for one more year.

Also, Lundbeck acquired LifeHealth; FDA is seeking a director of its new tobacco regulation branch; Charles River Labs announces personnel changes; more...

Johnson & Johnson has agreed to pay $1.0 billion to acquire the assets and rights of the Alzheimer's immunotherapy program of the biopharmaceutical company Elan, form a new company with Elan based on the AIP program, and gain an 18.4% stake in Elan.

Advances in micellar catalysis, solid-state chemistry, catalytic asymmetric synthesis, and function-oriented synthesis for natural products represent noteworthy developments in green chemistry that can be applied to the synthesis of active pharmaceutical ingredients.

Also, Wyeth and Catalyst sign agreement; FDA seeks public opinion about tobacco regulation; Catalent appoints VP of quality and regulatory affairs; more...

This week, the US Pharmacopeial Convention and the Vietnamese Pharmacopoeia Commission signed a memorandum of understanding that will help ensure the safety of Vietnamese medicines.

REMS to improve the safe use of opioids may lead to controls on other high-risk medicines.

The regulators are doing it. But industry's fear of sharing information may leave them behind.

Is it good policy to pay for bad behavior?
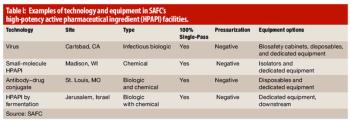
High-potency manufacturing of active pharmaceutical ingredients is a growing and specialized capability.

Traditional Chinese Medicine is widely used, but questions persist regarding its regulatory status.

Also, Adimab forms deals with Merck and Roche; Manhattan Pharmaceuticals' CEO and president steps down; more...

Generic-drug and specialty pharmaceutical manufacturer Watson Pharmaceuticals (Corona, CA) agreed to acquire the generic-drug company Arrow Group for $1.75 billion in cash and stock.

Following the 2008 launch of the European Fine Chemicals Group (EFCG) Voluntary Guidelines (VGs) to promote supply-chain security, the group has now announced the official launch of an assessment template that will allow fine chemicals customers and suppliers to assess and implement this new set of recommendations.

Also, FDA debars clinical investigators; Jubilant Organosys forms deal with Endo Pharmaceuticals; AMRI makes changes to its India management team; more...

The Subcommittee on Health of the US House of Representatives Energy and Commerce Committee held hearings last week to discuss the findings of a report by the Federal Trade Commission (FTC) that examined the competitive effects for follow-on-biologics (FOBs).

Also, TorreyPines Therapeutics to liquidate assets and dissolve company; EU's competition services to examine Pfizer/Wyeth merger; Akorn appoints Raj Rai interim CEO; more...

The European Commission's (EC) Directorate-General for Enterprise and Industry (EC-DG Enterprise) announced last week that it will not continue preparing a commission directive on good manufacturing practices (GMPs) for certain excipients.

The US biotechnology industry reached aggregate profitability for the first time ever in 2008, representing the only bright spot in an otherwise dismal year for biotechnology financing and performance.

Consumer-care products, electronics, and select industrial firms comprise AMR Research's Supply Chain Top 25 for 2009, offering an opportunity for the pharmaceutical industry to examine best practices in supply-chain management.

Members of the House Committee on Energy and Commerce, Chair Emeritus John D. Dingell (D-MI), Chair Henry A. Waxman (D-CA), and Reps. Frank Pallone (D-NJ), Bart Stupak (D-MI), Diana DeGette (D-CO) and Betty Sutton (D-OH) released last week a discussion draft of the Food Safety Enhancement Act of 2009.

Also, WACKER expands Iowa facility; EMEA releases a Q&A document for PIPs; Metrics consolidates quality operations; more...
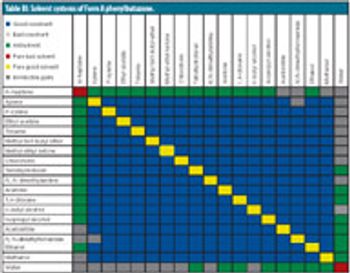
The authors describe the importance of a rapid and an abbreviated screening strategy in initial solvent screening. This article contains bonus online-exclusive material.

Misleading the public about their investments-be it money or medicine-is unacceptable.