
Exelixis And XenoPort Announce Job Cuts; GSK Dedicates India Facility; And More.

Exelixis And XenoPort Announce Job Cuts; GSK Dedicates India Facility; And More.

Astellas Pharma Makes Bid for OSI Pharmaceuticals; Ringo Retiring From Pfizer; And More.

Could President Obama's tax reform, which is targeted at reducing outsourcing, endanger India's contract-services industry?

Emerging markets remain an important element in the strategies of pharmaceutical companies and their suppliers.

Pre-Interphex 2010 Product Releases.
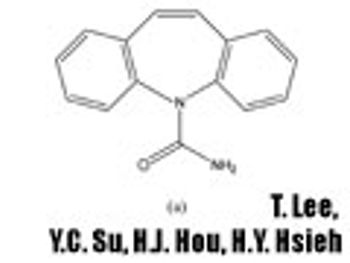
In Part I of this article, the authors describe the materials and methods used in developing a screening strategy to accelerate the preparation and characterization of spherical agglomerates by spherical crystallization.

To best carry out the vision of Hatch-Waxman, Congress must act now on biogenerics.

Contract manufacturers report improving business conditions, but will they continue?

Analysis of the opportunities and challenges in the biosimilars market.

From last-minute product inserts to putting out fires, close calls are a common occurrence.

If both sides of the aisle don't agree on even mild healthcare reform soon, the bill could die out.
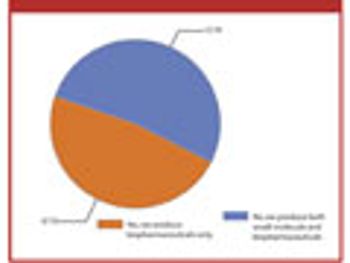
The second annual Pharmaceutical Technology Bioprocessing Survey offers a snapshot of the industry following 2009's megamergers.

Merck To Eliminate 15,000 jobs; Tauzin Stepping Down At PhRMA; And More.

RUSNANO, a corporation established by the Russian government to foster the development of nanotechnology, launched a project last week to manufacture targeted-delivery nanodrugs to treat malignant neoplasms.

The Society for Chemical Manufacturers and Affiliates offered its support for a Senate proposal to extend to 2015 current chemical-security standards, the Chemical Facility Anti-Terrorism Standards, which are set to expire this year.

Abbott Completes Acquisition Of Solvay; Merck Names President of Consumer Healthcare; And More.

Personalized medicine is still a nascent area for pharmaceutical companies, but several large companies recently reported developments in this area.

GSK Chief Executive Andrew Witty outlined the company's strategic priorities for 2010, which include further diversification into emerging markets, consumer healthcare, and vaccines.

Margaret Hamburg, commissioner of the US Food and Drug Administration, unveiled a new program to improve the efficiency of import inspections.

VaxGen Shareholders Reject OXiGENE Merger; Roche Creates Research Hub In Singapore; And More.

Pfizer's first updated pipeline since its acquisition of Wyeth includes fewer projects than before and is targeted to specific diseases.

Cephalon Buys Mepha; BASi's CEO Retires; and More.

AstraZeneca announced this week that it plans to undertake further restructuring in its research and development operations, resulting in the elimination of 3500 jobs.
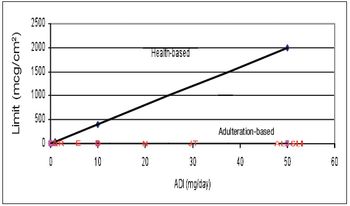
The author discusses how the use of a visible residue limit has made the 10-ppm cleaning limit obsolete in many applications.

Sharing too much-or too little-information can have disastrous onsequences.