
Company and People Notes: sanofi aventis forms pact with Micromet; Laureate Pharma adds members to its business team.

Company and People Notes: sanofi aventis forms pact with Micromet; Laureate Pharma adds members to its business team.

A panel of industry and regulatory experts, including FDA, discuss efforts to secure the global pharmaceutical supply chain. This article contains bonus online-exclusive material.

While Congress debates hundreds of healthcare plan proposals, perhaps we, the public, can get in the game too.

Functionalized supramolecular catalysts and an enantioselective route to unnatural amino acids are some recent developments.

A recent book reminds readers that small-molecule chemistry has enabled advances in biotechnology.

FDA's Edwin Rivera-Martinez on dodging supply-chain challenges.
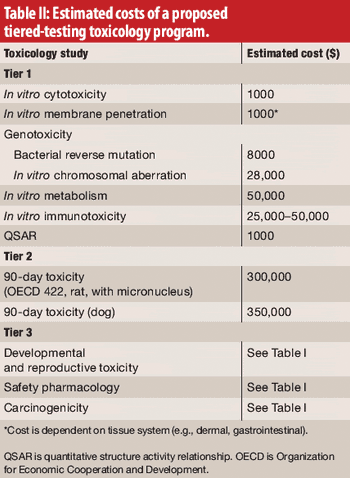
The authors, representing the International Pharmaceutical Excipients Council, propose a new evaluation procedure, including tiered toxicology testing for excipients.

A Conversation with Otonomy

Cargill has recently announced a number of innovations connected to its range of polyol excipients.

The House Energy and Commerce Committee approved H.R. 2868, the "Chemical Facility Anti-Terrorism Act of 2009," a measure to modify and codify existing requirements of the Chemical Facility Anti-Terrorism Standards (CFATS) program.

Also, Astellas and Medivation sign development pact; WuXi PharmaTech appoints VP of business development, more...

The global API market has expanded from $69 billion in 2004 to more than $90 billion in 2008, according to a report from the Italian Chemical Pharmaceutical Association (CPA); in particular, the report noted a rapid growth rate for generic APIs.

Company and People Notes: SurModics forms agreement with Roche and Genentech; Hospira names Daphne Jones senior VP and chief information officer; more...

Following the completion of its $68-billion acquisition of Wyeth (Madison, NJ), Pfizer (New York) began joint operations of the combined company last week.

The European Fine Chemicals Group (EFCG) gave an update of some of its activities at a press conference held at CPhI Worldwide (Spain) last week, and revealed some disturbing facts regarding counterfeit APIs, which the group is particularly concerned about.

Company and People Notes: Bayer Schering Pharma forms collaboration with Compugen; Takeda San Francisco appoints VP of process science; more...

The US Food and Drug Administration issued an alert to healthcare professionals of a change in heparin manufacturing that is expected to decrease the drug's potency.

The 2009 Nobel Prize in Physiology or Medicine was awarded to three US researchers for the discovery of how chromosomes are protected from degradation by telomeres and the enzyme telomerase during cell division.

GlaxoSmithKline (London), Novartis (Basel, Switzerland), sanofi Aventis (Paris), and Baxter International (Deerfield, IL) recently provided updates as to the development, manufacture, or shipment of pandemic (H1NI) vaccines.

Company and People Notes: GSK forms joint venture with China-based Jiangsu Walvax Biotech; Sigma-Aldrich appoints VP and board member; more...

AAPS President offers hope and solutions for the industry's challenging future.

The nation's healtcare system needs an overhaul, but it has to be done right.

New nanotechnology-based delivery systems offer promise in drug delivery, particularly for anticancer therapeutics.

Applying a moisture protective barrier to tablet cores can help prevent degradation caused by ambient moisture.

The biopharmaceutical company Seattle Genetics (Bothell, WA) introduced a sugar-engineered antibody (SEA) technology that is designed to increase the potency of monoclonal antibodies through enhanced effector function.