
Capacity expansions and new products meet needs for inhaler and injector systems.

Capacity expansions and new products meet needs for inhaler and injector systems.

With the enforcement of the European Directive 2011/62/EU relating to medicinal products for human use regarding falsified medicinal products, new requirements are introduced for active substances. The new regulation requires among other things, documentation of the supply chain traceability.

On-demand drug release has the potential to reduce the side effects associated with the over-dosage of drugs, particularly for highly potent anti-cancer therapies.

3M Drug Delivery will manufacture transdermal patch for ProStrakan.

Formulation and process considerations for ethyl cellulose aqueous dispersion in sustained-release applications.

Kurt Lumsden, Director Client Services at Perceptive Informatics, a subsidiary of PAREXEL, discusses regulatory requirements for the drug accountability process.
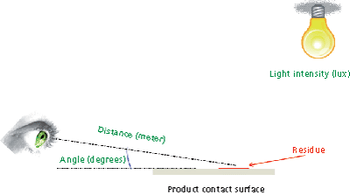
The authors evaluated a variety of materials of construction (MOCs) and found that visible residue limits (VRLs) were higher on some MOCs than on stainless steel. The optimal viewing conditions were dependent on the MOC and the viewing background. The risk of a cleaning failure due to visual failure for different MOCs can be mitigated or eliminated using complementary cleaning validation studies.

Through its educational and networking opportunities, the American Association of Pharmaceutical Scientists plays an important role in partnering throughout the drug- development and commercialization process.

Researchers from the University of Bradford, UK, have developed a solvent-free, continuous method to manufacture the more soluble and bioavailable form of artemisinin. The metastable form produced using high-temperature extrusion has been shown to have greater stability and longer shelf-life.

Cedarburg Hauser Pharmaceuticals has upgraded its API plant in Wisconsin.

J&J's Janssen and PATH partner to improve drug formulation that could help prevent HIV infection.

Molecular Profiles has secured a major pan-European formulation project, in which the company will be responsible for developing a topical and an oral formulation for Telormedix's psoriasis products, TMX-302.

Pfizer advances its pipeline of antibody-based therapies using a host cell line that combines engineered glycosylation technology and enhanced gene-expression.

Shamrock Medical Solutions Group, a drug repackaging and distribution company, repeatedly failed to comply with good manufacturing practices.

Unilife grants Sanofi long-term exclusivity for the use of Unilife?s prefilled syringe with Sanofi?s Lovenox.

FDA updates guidance to reflect advances in technology.

New partnership aims to eliminate months from the typical transition time required to move chemistry from the laboratory into commercial applications.
Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

The new subcutaneous formulation of Herceptin can be administered six times faster than the standard intravenous formulation.

Are strategic partnerships in clinical research a model for CMC services?
The adoption of quality by design in small-molecule drug development and manufacturing continues to evolve as the industry seeks ways to augment process understanding for APIs

The advantages of using an automated powder dispensing system in a ventilated balance enclosure for efficient handling and effective containment of potent compounds are discussed.
Developments involve stereoretentive cross-coupling, enantioselective alcohol silylation, strategies for amplifying signals in circular dichroism spectroscopy, and a synthetic route for the natural product ingenol.
Industry experts share their views on the outsourcing model and the current and future direction of contract chemical API manufacturing.
Commercial-scale amide formation and an improved process route for a tetracycline derivative are some recent developments in API synthesis.