
Company receives notice from FDA for not fully investigating foreign particles in APIs and finished products made from its facility in Ingelheim am Rhein, Germany.

Company receives notice from FDA for not fully investigating foreign particles in APIs and finished products made from its facility in Ingelheim am Rhein, Germany.

Dynamic testing and advances in shear testing provide better insight into powder physical properties and external variables that affect powder behavior.

Access to extremely low and consistently controlled reaction temperatures can improve product yields and selectivities while also reducing costs.

New platform technologies and polymer chemistries may facilitate self-administration, longer-term delivery, and targeted delivery of parenteral drugs.

Applications of ZFN technology in biopharmaceutical cell-line engineering.

While there are those who want combination products to be controlled by a centralized pharmaceutical-type approval system, the majority of the medical technology industry wants to retain a decentralized device-focused approach.

The rejection by India's Supreme Court on Novartis' Glivec/Gleevec (imatinib mesylate) and other recent case law raise important issues on patent strategies for solid forms.
Parenteral drug delivery offers a variety of challenges but also opportunities. The author examines recent developments in nanotechnology-based drug delivery and other advances in injection-based drug delivery.
The authors describe a holistic and integrated approach to focus on the linkage of the prefilled syringe with the four phases of product design, development, operation, and control.

Researchers recently developed a drug-delivery system to mitigate problems associated with jet-injection drug delivery, and also improved on the design and operation of microscale actuators as a possible drug-delivery method.

Because peptoids are relatively easy to synthesize, have tremendous sequence diversity, and are more proteolytically stable than peptides and proteins, they have significant potential for use as APIs, drug-delivery agents, and in numerous biomedical applications.

Oval Medical Technologies, an autoinjector company based in Cambridge, UK, reported that a variety of highly viscous solutions have been successfully delivered through a 25-gauge thin-wall needle, in less than 7 seconds, using its innovative autoinjector. The technology provides solutions to problems in the industry for drug containment and the end user.

Shimadzu's LCMS-8040 combines newly improved ion optics and collision cell technology with proprietary ultrafast technologies.

The challenge of developing orally inhaled nasal drug products (OINDPs) is complicated by the interplay between drug-delivery devices and formulation.

An introduction to the upcoming Interphex panel--Lessons Leaned: Successes and Challenges in Implementing Quality by Design.? Moderator: Jennifer Markarian, manufacturing editor, Pharmaceutical Technology. Panelists: John Lepore, senior director of Chemical Process Development and Commercialization for Global Pharmaceutical Commercialization at Merck and Co. Chris Moreton, FinnBrit Consulting Jonathon Thompson, senior manager of Compliance Services Consulting at Invensys Operations Management

The parties partner for evidence-based formulations for emerging markets

Access to simpler and more direct methodologies for the incorporation of fluorinated substituents remains an unmet need in pharmaceutical synthesis. An analysis of recent trends in fluorination chemistry.
Continuous flow chemistry offers potential for greater control, improved safety and environmental profiles, and efficient chemical transformations.

Contract API manufacturers proceed with select investment in capacity and service additions.
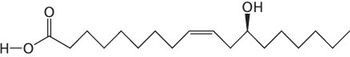
This article summarizes the development and modernization of the United States Pharmacopeia-National Formulary (USP-NF) fixed-oil excipient monographs. This article contains bonus online-exclusive material.

A Q&A with Tony Hitchcock, head of manufacturing at Cobra Biologics.

The European Union authorities are stepping up their efforts to incorporate quality-by-design principles into their regulations and guidelines.

Keith Bader, senior director of technology at Hyde Engineering + Consulting discusses his presentation ?Establishing a design space: cleaning process development and validation,? which will be presented at Interphex 2013

The 2013 Excipient Information Package (EIP) User Guide is now available for free download from the International Pharmaceutical Excipients Council (IPEC)-Americas.

Collaboration provides pharmaceutical industry customers with access to enzyme expertise and assets resulting in cost-effective, green processing at all phases of development, from preclinical to commercial manufacturing scale.