
CDER withdraws some outdated guidance documents and makes plans to finalize others.

CDER withdraws some outdated guidance documents and makes plans to finalize others.

Rapid screening of critical API properties can quickly identify the best approach for increasing bioavailability.

A simple, rapid analytical method for the determination of palladium in pharmaceutical production samples will speed up development and optimization of reactions involving palladium catalysts.

The companies initially focused on five biosimilar products.

Adamis Pharmaceuticals agrees to license and potentially acquire 3M's Taper Dry Powder Inhaler technology.
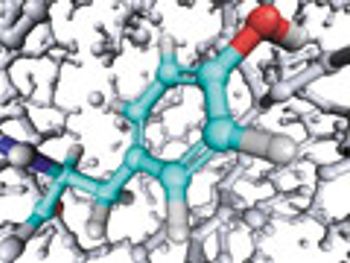
Stapled peptides offer promise to enable cell permeability, binding to therapeutic targets, and modulation of biological pathways.
Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

Nanomedicines have been authorized by European licensing agencies for more than 30 years but are still posing regulatory difficulties.

View companies' outsourcing profiles from PharmTech's 2013 Outsourcing Resources Fast Locator Index in the following categories.

Successful product development and technology transfer between a CMO and the sponsor company requires a multifaceted collaborative approach. The author, Marga Vines from Grifols International, analyzes a project for an injectable drug for which a new drug application was submitted to FDA.

Industry groups offered perspectives on the strengths and weaknesses of the European Falsified Medicines Directive.
External manufacturing plays a crucial role in pharmaceutical companies’ supply strategy. The author examines market trends for the captive and merchant global market for active pharmaceutical ingredients and intermediates.

Australian team develops method for making ultrafine particles for more efficient drug delivery.

The report highlights a need for greater third party certification to ensure GMP vigilance.

Novel, atom-efficient routes to amides from esters and carboxylic acids for more sustainable manufacturing.

New routes enable the efficient synthesis of enantiopure sulfinamides and structurally and sterically diverse P-chiral phosphine oxides.
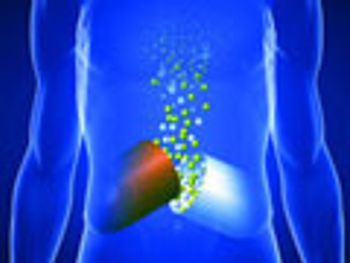
Osmotic systems offer versatility for delivering drugs with varied properties and dosage requirements.

Claudia Roth, President of Vetter Development Service USA, discusses trends in single-use technology for clinical manufacturing.

Falsified Medicines Directive requires imported APIs to have written confirmation of GMP standards.

Contract service providers expand capabilities in API and finished product manufacturing to meet demand for high-potency drugs.

GE Healthcare's partnerships with iBio and Brazil's Bio-Manguinhos/Fiocruz for a new plant-based multipurpose biopharmaceutical and vaccine manufacturing facility move plant-based protein production to the next level.

The US and EU move forward with measures to fortify the pharmaceutical supply chain.

Cocrystals can enable the formulation of solid dosage drugs, but the FDA's final guidelines have left concerns about how their use could impact development timelines, the drug product manufacturing process, and the intellectual property position of products containing cocrystals.

Eisai has launched a new dry syrup formulation of its Alzheimer's disease treatment Aricept (donepezil hydrochloride) in Japan.

Until recently, glycan analysis has been a slow, labor-intensive process more widely used late in bioprocess development. New high-throughput methods are changing that.