
Continued process verification for a cleaning validation program begins once the validation study is complete.
Richard J. Forsyth is principal consultant with Forsyth Pharmaceutical Consulting, 907 Shamrock Ct, Royersford, PA 19468, tel. 484.535.1688, forsythpharmaconsulting@gmail.com.

Continued process verification for a cleaning validation program begins once the validation study is complete.

A robust selection of which product(s) and equipment to validate for cleaning is the cornerstone of a successful cleaning validation program. A strategy for selection of products and equipment for cleaning validation is presented.

Swab recovery parameters are reviewed in detail to define best practices and highlight common mistakes to assure successful recovery studies using a risk-based approach.

This article presents recommendations based on a program that was set up to qualify members of a large, diverse team at one oral solid-dosage-form manufacturing facility.
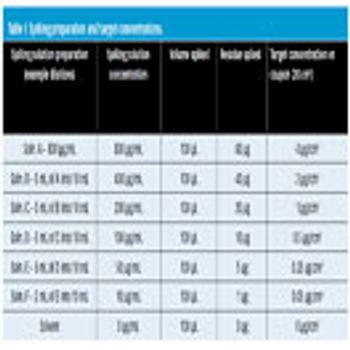
Visible residue limits have been shown to be a valuable tool in validated cleaning validation program.
An integrated approach can improve the efficiency of cleaning validation studies.
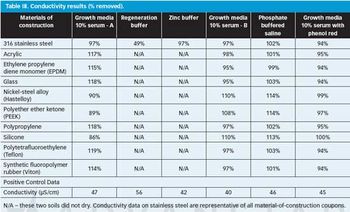
The authors look at the cleanability of pharmaceutical soils from a variety of materials of construction to determine the relative ease of cleaning and explore potential grouping strategies as part of a comprehensive cleaning validation program.

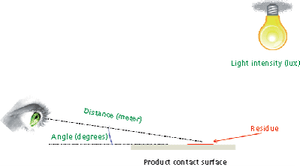
Different approaches for qualification of personnel for visual inspection of residues on equipment surfaces are reviewed.
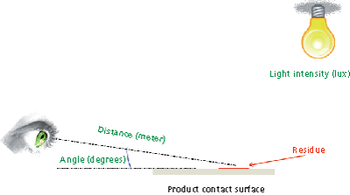
The authors evaluated a variety of materials of construction (MOCs) and found that visible residue limits (VRLs) were higher on some MOCs than on stainless steel. The optimal viewing conditions were dependent on the MOC and the viewing background. The risk of a cleaning failure due to visual failure for different MOCs can be mitigated or eliminated using complementary cleaning validation studies.

The author challenges current detection methodologies.

Regulatory agencies expect companies to establish and monitor clean equipment- and dirty equipment-hold times for manufacturing equipment as part of their cleaning validation program.

An adulteration limit of 100 ?g/25cm? (4 ?g/cm?) was proposed for pilot-plant facilities. The dynamic changes in equipment, formulation, and residue determination made implementation of a constantly changing, calculated adulteration limit impractical. A single adulteration limit was simpler to communicate and document, making compliance achievable. The limit would be used only after it was determined to be lower than a health-based evaluation and a visual-cleanliness assessment.

Published: January 2nd 2022 | Updated:

Published: August 3rd 2021 | Updated:

Published: January 2nd 2014 | Updated:
Published: October 2nd 2013 | Updated:

Published: March 2nd 2011 | Updated:

Published: April 2nd 2008 | Updated: