
The authors examine the use of various grades of direct-compression mannitol in direct-compression tableting process to evaluate the content uniformity of micronized APIs and excipients in a solid-dosage formulation.

The authors examine the use of various grades of direct-compression mannitol in direct-compression tableting process to evaluate the content uniformity of micronized APIs and excipients in a solid-dosage formulation.

A Q&A with James Ingebrand, Vice President and General Manager of 3M Drug Delivery Systems Division, on recent industry trends.

Particle-engineering technologies, such as crystal design for crystallization and producting cocrystals, particle-size reduction, and amorphous solid dispersions, help to optimize delivery of a drug.

The long-awaited patent cliff that has loomed in the pharmaceutical industry for years has arrived in earnest in 2012, with more than $40 billion in 2011 brand sales facing loss of exclusivity.
This study examines the effect and interaction of variations in hypromellose physicochemical properties.
Fixed-dose combination drug therapies give rise to innovation in solid-dosage formulations and manufacturing.
Researchers have developed injectable, reformable, and spreadable hydrogels capable of delivering sustained release of the proteins they contain for up to six months.
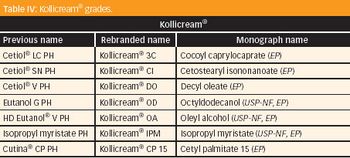
Solubilizers play an important role in dissolving poorly soluble molecules. As the number of poorly soluble lipophilic and/or hydrophobic molecules increases-whether as "brick dusts" or waxy substances-the industry is struggling to identify the appropriate lipophilic excipients (surfactants, solubilizers, solvents or polymers) that can be used to develop such poorly soluble formulations into solid dosages and other forms of pharmaceutical products.

A review of taints and odors in the pharmaceutical and consumer healthcare industries.

Advances in targeted drug delivery and customized release profiles are key industry goals.

Ties between the biotechnology industry and university research are crucial.

Industry and academia advance novel approaches for achieving enanioselectivity.

USP optimizes identification tests and impurities procedures.

Development of viable dosage forms for poorly water-soluble compounds continues to be a significant challenge for formulation scientists, and insufficient bioavailability of such compounds may result in development delays or failures.

The authors describe a solid form technology platform used to optimize salt selection, cocrystallization identification and modification, or the development of a free form.

Pfizer has two manufacturing facilities in Germany for high-potency manufacturing, respectively in Freiburg and Illertissen. Pharmaceutical Technology's Executive Editor Patricia Van Arnum visited the facilities and spoke to the company about the design and operation of these facilities.
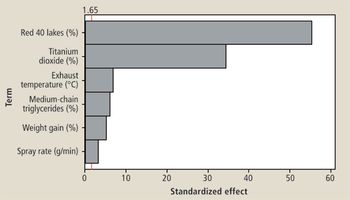
The authors describe a QbD study that was performed to optimize a coating system.

A Q&A with BASF moderated by Patricia Van Arnum.

Rob Blanchard and Clive Roberts discuss the issues surrounding tablet sticking.
The authors explain chemical transformations that are achievable through certain biocatalytic routes.

An industry roundtable representing Metrics, Cambrex, Carbogen Amcis, Euticals, Ferro Pfanstiehl, and SAFC.

Approaches to scaling up API syntheses center on ways to optimize process conditions and operability.
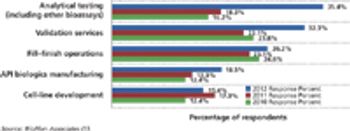
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.
Enhancing bioavailability can be achieved through hot-melt extrusion or spray drying. Patricia Van Arnum interviews Bend Research to find out more about when to use each technique.

Meticulous system configuration can prevent machines from taking over.