
The author discusses various aspects of the hot-melt extrusion process and outlines a practical approach to scale-up.

The author discusses various aspects of the hot-melt extrusion process and outlines a practical approach to scale-up.

Charlie Martin, Leistritz Extrusion, describes hot-melt extrusion manufacturing processes and advances.

The author examines the effect of compaction force, gap width, and sieve setup on granule size.

A study investigated the influence and correlation of critical process parameters for the optimization of in-process curing.

The selection of excipients is important in generic formulations due to the impact it has on the risk and performance of generic drugs.
Increasing potency and growing interest in antibody-drug conjugates are creating challenges for manufacturers of HPAPIs.

The international pharmaceutical industry is changing its approach to R&D and is increasingly relying on outsourcing for drug discovery.

Kurt Lumsden, Director, eCDS Client Services at PAREXEL Informatics, discusses eClinical supply-chain management.

Mark D. Kramer talks about combination products from a regulatory perspective and explains the implications of FDA's final rule on current GMP requirements for combination products.

As Europe strives to firmly incorporate quality-by-design principles, there are several key issues that still need to be addressed.
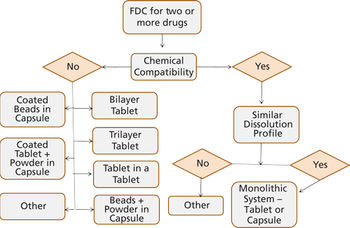
Anil Kane from Patheon spoke to Pharmaceutical Technology about the challenges in the development of FDCs and discussed how to integrate quality-by-design (QbD) in the manufacturing process of FDCs.

Advanced drug delivery technologies can increase efficacy and safety, extend patent lives, and provide competitive differentiation for biopharmaceuticals.

Adocia has reported positive results for the first clinical trial of its combination insulin formulation of fast- and long-acting insulin in patients with type I diabetes. Results showed that BioChaperone Combo provided both short- and long-term control of blood glucose in type I diabetic patients, with a faster onset and a longer duration of action.

Novartis has launched the prefilled syringe formulation of Lucentis (ranibizumab) in Germany, with other markets to follow throughout 2014. The prefilled syringe formulation has been specifically designed for intraocular injection to enhance patient safety and convenience for the treating clinician.

Looking to improve patient access to new medicines, EMA creates a pilot project to explore an adaptive licensing approach with real medicines in development.

Agencies extend successful pilot program to further harmonization of QbD topics.

Accelerated testing and production create challenges in documenting product quality.

With nanomedicines on the rise, a new class of non-biological complex drugs (NBCDs), which include nanosimilars, has emerged. As drug regulators are faced with the challenge of defining a framework to ensure the safe introduction of the follow-on nano-therapeutics, Stefan Muhlebach explains why NBCDs cannot be assessed using the standard generic or biosimilar approaches.
The commercial availability of an increasing diversity of enzymes has led to the growing use of biocatalysts for API synthesis.

Drug formulators are looking for new excipients to address solubility and bioavailability issues and extend patent protection, but there are hurdles to using novel, specialized ingredients.

CMOs may find opportunities in alternative expression services.
Experts discuss factors affecting drug delivery to the lungs and key considerations when developing inhalation formulations.
Automated sample handling, advanced glycan analysis, and specially designed columns are help speed up confirmation of the biosimilarity.

Recent regulatory initiatives designed to secure the global pharmaceutical supply chain will directly impact the global supply chain and API manufacturers.
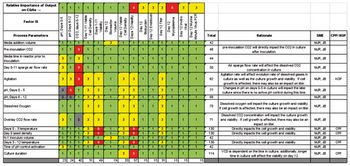
Traditional project decision-making is compared with a QbD approach.