
McNeil-PPC pleads guilty in connection with adulterated infants' and children's over-the-counter liquid medications.

McNeil-PPC pleads guilty in connection with adulterated infants' and children's over-the-counter liquid medications.

Protecting workers, patients, and the environment requires advanced technologies.

Assessing risk factors is key to implementing the new ICH Q3D guidelines for elemental impurities.

While the skin offers an alternative route of administration for local and systemic drug delivery, developing semi-solid dosage forms can be a challenge.
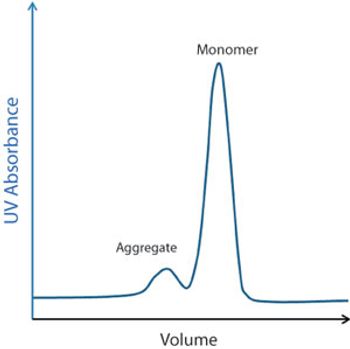
Several chromatographic resins are available for downstream purification.

It is vital that companies involved in the manufacturing and handling of cytotoxic drugs ensure that staff are given the highest possible levels of protection.

FDA approved Actavis’ antibiotic Avycaz designed to combat drug-resistant bacteria.

Turing Pharmaceuticals announced the launch of its company, the acquisition of three assets from Retrophin, and a new senior management team.

Bristol-Myers Squibb announced that it reached an agreement to acquire Flexus Biosciences and has entered into a $309-million partnership with Rigel Pharmaceuticals.

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.

Celgene announced that its drug, Revlimid, gained FDA approval for the treatment of newly diagnosed multiple myeloma.

The all-synthetic 3M Emphaze AEX Hybrid Purifier contains both an anion-exchange nonwoven media and a fine-particle, bioburden reduction membrane.

Amgen announced that it met primary and secondary endpoints in its biosimilar evaluation of adalimumab for the treatment of rheumatoid arthritis, when compared to Humira.

AstraZeneca announced that it would strengthen its respiratory pipeline with the acquisition of Actavis’ branded respiratory business in the US and Canada.

The Medicines Company announced that it acquired all the remaining equity of Annovation Biopharma, including its novel anesthetic ABP-700.

Sanofi launched a rapidly absorbed, short-acting inhalable insulin in the US to help control type 1 and type 2 diabetes.

Mylan announced a partnership with Theravance Biopharma to develop and commercialize TD-4208, a novel investigational COPD treatment.
Resolution technologies remain crucial for commercial-scale chiral API production.

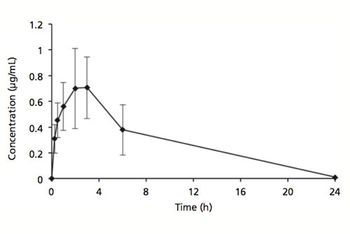
Formulating an injectable solution containing both hydrophilic and hydrophobic drugs is a challenge.
Working with biological matrices and understanding the intended use are crucial.
New guidelines and best practices may lead to improved quality and reduced recalls due to visual defects.
Whether outsourcing or developing cell therapies in-house, success demands a focus on quality, cost of goods, and sustainability from the start.

CMO executives share their opinions on where outsourcing is going and what is driving market change.
After launching a new mammalian cell platform, FUJIFILM Diosynth Biotechnologies U.S.A., has acquired fast-track vaccine manufacturing knowhow and a major presence in Texas’ emerging biocorridor with Kalon, its first acquisition.