
As the prevalence of falsified medicines continues to increase, Switzerland is taking measures to secure its supply chain such as the implementation of serialization.

As the prevalence of falsified medicines continues to increase, Switzerland is taking measures to secure its supply chain such as the implementation of serialization.

A review of how mergers and acquisitions have shaped the pharmaceutical industry landscape.

With the rising threat of antibiotic resistance and a diminishing R&D pipeline for novel drugs, Europe must step up its game to address this growing problem.

The international pharmaceutical industry is changing its approach to R&D and is increasingly relying on outsourcing for drug discovery.

Many pharmaceutical companies in the UK have adopted a direct-to-pharmacy distribution model, which enables companies to more tightly control their supply chains.

Phase 0 studies, though not widely accepted, could effectively bridge the gap between preclinical and Phase I studies, and reduce the number of drug failures owing to differences in drug behaviour between animals and humans.

Despite being a historically strong performer in the pharmaceutical arena, Germany is now underperforming compared with the growth of other European markets.

Though many advantages are associated with the European Clinical Trials Directive, complexities have emerged since its introduction in 2004. Since 2007, efforts have been made to raise the issues and address the negative impact of the Directive.

In 1995, pharmaceutical regulation in Europe underwent a dramatic change with the emergence of the European Medicines Agency. The agency's 15-year history has been eventful, with it having to adapt to a changing regulatory landscape and new expectations from those relying on it for guidance.

Could a collaborative approach involving both public and private parties offer a solution to Europe's lack of innovation?

Although representing a huge market, the EU also presents problems that hamper profitability- particularly when it comes to differing price regulations across member states.

Population ageing is an issue often discussed in the media, but its impact on all facets of life has been poorly characterized.

Even though the pharmaceutical industry has come to recognize the importance and associated benefits of using computers, evolving computer-based simulation technology has still to make a suitable impression.

Rare diseases represent an important area of unmet medical need.

Modern medicines have dramatically improved the health of millions of people worldwide, but the healthcare situation in industrialized countries is a stark contrast to that in the least developed countries where neglected diseases are still prevalent.

With a new head of the FDA expected to be announced imminently, the pharmaceutical industry waits to witness the changes that will inevitably accompany the new appointment. These changes could, however, also impact the rest of the world's pharmaceutical markets.

The productivity of the pharma industry has been declining for several years. Could biotechnology reinvigorate drug development?

How will the world's dominant pharmaceutical market fare under the new US president?

Together, Europe and the US account for more than 70% of the global pharmaceutical market, and the growth of these markets is heavily dependent on distribution systems.

All commercial sponsors want to maximize their profit and, with a population close to 500 million, the EU is an enormous potential marketplace that they simply cannot ignore!

Despite high ambitions for its pharmaceutical and biotech sectors, South Korea still faces a number of hurdles against competing internationally.


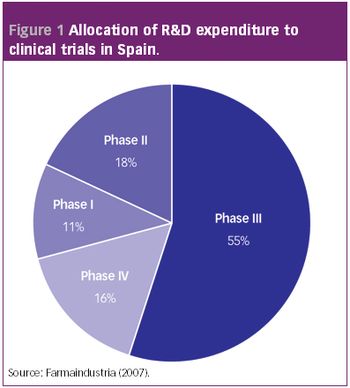
Although overall pharmaceutical industry investment in R&D in Spain is growing at a slower annual level since 2004, investment in clinical trials remains strong.
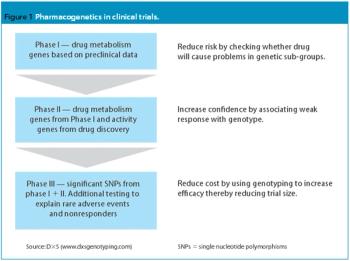
The completion of the Human Genome Project in 2003 led to a flurry of predictions regarding the application of pharmacogenomics to drug development. With US and European regulatory authorities finally on the verge of issuing guidance on the use of pharmacogenomics, drug development is all set to change.

Driven by a rapidly ageing population and a known high consumption of pharmaceuticals, the French pharmaceutical market has always appeared buoyant.
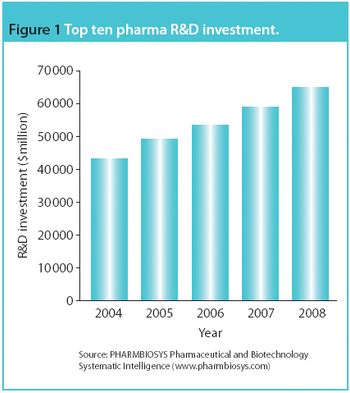
Although the CRO may not wish to draw attention to weaknesses in the intended trial, failing to do so is a great disservice.
Although it has recently been surpassed by Mexico in terms of value, the Brazilian pharmaceutical market remains of key importance to companies establishing themselves in Latin America. The Brazilian government has focused heavily on improving the healthcare system and this should lead to long-term benefits for its citizens. The Ministry of Health has also attempted to decentralize the management of the healthcare system to more regional and local levels. This has been necessary to account for the different healthcare priorities in different parts of the country.
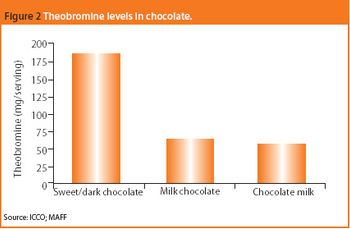
Could compounds in chocolate yield new pharmaceutical approaches to major disorders?

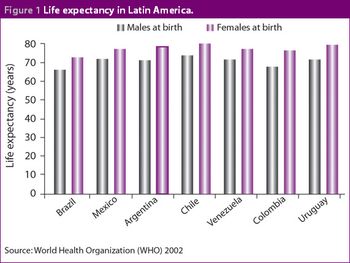
Latin America has become a promising region for the pharmaceutical industry in terms of both R&D and sales. Nowhere has this been more apparent than in Mexico, which has become a top priority market for many of the major multinational pharmaceutical companies. Furthermore, the Mexican government is increasing its investment in healthcare resources and there is a strong, growing demand from the population for access to newer and better medical treatments.

Published: April 2nd 2014 | Updated:

Published: April 1st 2010 | Updated:

Published: August 1st 2010 | Updated:

Published: February 1st 2010 | Updated:

Published: March 1st 2010 | Updated:

Published: June 1st 2010 | Updated: