
Combination products have been researched and administered for many years, some successfully and others not.

Combination products have been researched and administered for many years, some successfully and others not.

Combining drugs with synergistic mechanisms of action yields some indisputable benefits; improved efficacy, reduced dosing, enhanced patient compliance, to name just a few.

Parteck ODT is a newly introduced ready-to-use excipient for fast melt tablets.

Company and People Notes: UCB and Novartis form agreement; AAIPharma appoints VP of regulatory affairs; more...

Researchers at the University of Illinois (US) have developed a cancer drug delivery system that reportedly kills target tumour cells, spares healthy cells and has effects that can be reversed to halt potentially hazardous side effects.

Also, FDA publishes draft guidances of two ICH Annexes; EMEA sets format for compliance advice; more...

Also, XCELERON and GSK form agreement; Millipore appoints VP of life sciences; more...

The US Food and Drug Administration?s Draft Guidance for Industry?Process Validation: General Principles and Practices provides a life-cycle approach for validating pharmaceutical processes and aims to help pharmaceutical companies achieve consistently high product quality.

Uncovering the root cause of contamination and leveraging the collaborative learning loop of QbD -Aegis Whitepaper

Also: DSM's North Carolina facility receives SafeBridge certification; Dynavax CFO to retire; more...

Baxter International (Deerfield, IL), sanofi aventis (Paris), and Novartis (Basel, Switzerland) provided updates last week of their production and regulatory activities relating to preparedness in supplying the A(H1N1) pandemic influenza vaccine. Novartis also outlined its activities for providing seasonal flu vaccines.

Also, Pfizer forms two research agreements in China; NanoInk appoints John Kubricky to its scientific advisory board; more...
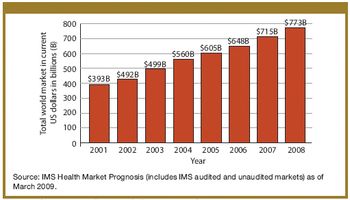
Biologics enhance their positions amidst slowing growth in the global and US markets.

Brief pharmaceutical news items for August 2009.

Biotech firms must first close the gaps between science and biology on the path toward QbD.
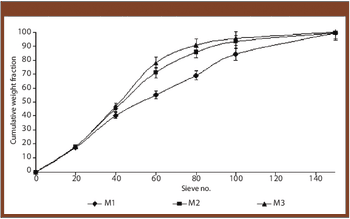
The authors prepared granules containing cinnarizine using polyethylene glycol 6000 as a melting binder and lactose monohydrate as hydrophilic filler. The effects of binder concentration and size were studied.

The author of a book about biopharmaceutical production includes irrelevant information.

Individuals and companies at the top seem to have no problem short-circuiting their success.

An ounce of contamination usually leads to a mountain of investigation.

The authors explain waivers and deferrals for pediatric studies of drugs and biologics as provided by the Pediatric Research Equity Act of 2007.

Researchers working on extending the shelf life of antibody drugs may find help in a computer model developed by a research team at MIT (Cambridge, MA).

Also, Isogen completes sterile facility; Dishman appoints head of generic API business; FDA releases final rule on authorized generic drugs and NDA submissions; more...

Also, Gilead and Tibotec form agreement; FDA approves 2009-2010 flu vaccine; Catalent appoints VP in packaging unit; more...

Also, Lonza and Medarex sign agreement; Covance appoints VP and chief scientific officer of global analytical services; more...

Also, Lundbeck acquired LifeHealth; FDA is seeking a director of its new tobacco regulation branch; Charles River Labs announces personnel changes; more...