
This week, the US Pharmacopeial Convention and the Vietnamese Pharmacopoeia Commission signed a memorandum of understanding that will help ensure the safety of Vietnamese medicines.

This week, the US Pharmacopeial Convention and the Vietnamese Pharmacopoeia Commission signed a memorandum of understanding that will help ensure the safety of Vietnamese medicines.

REMS to improve the safe use of opioids may lead to controls on other high-risk medicines.

The regulators are doing it. But industry's fear of sharing information may leave them behind.
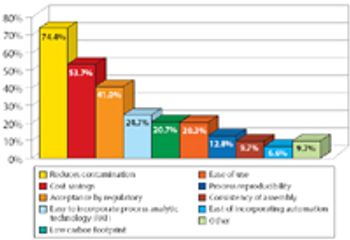
A Pharmaceutical Technology report looks at trends in biopharmaceutical manufacturing. This article contains bonus online-exclusive material.
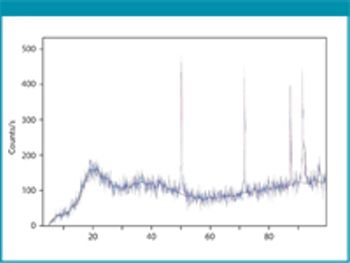
The authors modified gellan gum using microwave technology and showed it can be used as an excipient in tablet formulations.
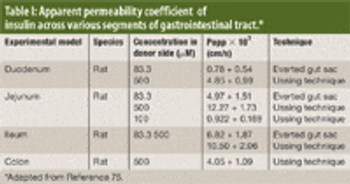
The authors review various oral drug delivery systems that have been explored to increase patient compliance for insulin.

Some GMP agents seem to find a way to squander time, money, and common sense.

Is it good policy to pay for bad behavior?
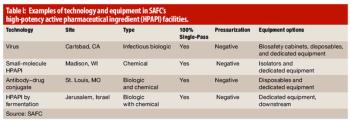
High-potency manufacturing of active pharmaceutical ingredients is a growing and specialized capability.

Traditional Chinese Medicine is widely used, but questions persist regarding its regulatory status.

A look at the true cost-drivers of cell-culture production.

Developers of low-dose drugs in solid oral dosage forms will find theoretical considerations and practical advice in a new book.
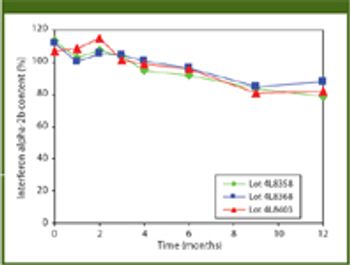
The authors describe a proprietary process for producing a stable, topical interferon alpha-2b formulation that can deliver large drug molecules into the skin or mucosa.
Cefaclor is a β-lactam cephalosporin antibiotic that has a wide particle size distribution. Because of the nonporous nature of the material, the specific surface area value accounts for a significant amount of fine particles possibly present in the samples under analysis.

Also, Adimab forms deals with Merck and Roche; Manhattan Pharmaceuticals' CEO and president steps down; more...

Also, FDA debars clinical investigators; Jubilant Organosys forms deal with Endo Pharmaceuticals; AMRI makes changes to its India management team; more...

The Subcommittee on Health of the US House of Representatives Energy and Commerce Committee held hearings last week to discuss the findings of a report by the Federal Trade Commission (FTC) that examined the competitive effects for follow-on-biologics (FOBs).

The emergence of influenza A (H1N1) and the efforts to provide vaccines to the vulnerable are timely examples of biopharmaceuticals' continuing importance.

Also, TorreyPines Therapeutics to liquidate assets and dissolve company; EU's competition services to examine Pfizer/Wyeth merger; Akorn appoints Raj Rai interim CEO; more...

The European Commission's (EC) Directorate-General for Enterprise and Industry (EC-DG Enterprise) announced last week that it will not continue preparing a commission directive on good manufacturing practices (GMPs) for certain excipients.

Consumer-care products, electronics, and select industrial firms comprise AMR Research's Supply Chain Top 25 for 2009, offering an opportunity for the pharmaceutical industry to examine best practices in supply-chain management.

Also, WACKER expands Iowa facility; EMEA releases a Q&A document for PIPs; Metrics consolidates quality operations; more...
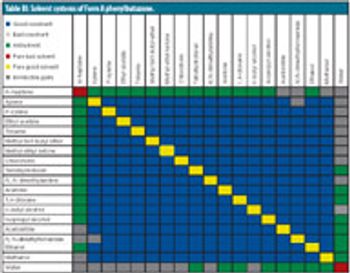
The authors describe the importance of a rapid and an abbreviated screening strategy in initial solvent screening. This article contains bonus online-exclusive material.
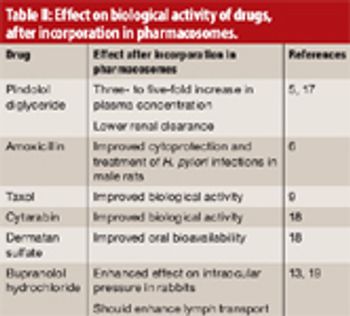
Pharmacosomes can pass through biomembranes efficiently and possess several advantages over traditional vesicular drug-delivery systems.

Misleading the public about their investments-be it money or medicine-is unacceptable.