
Obama's cost-containment and science-innovation initiatives need to overlap.

Obama's cost-containment and science-innovation initiatives need to overlap.

A review of recent product innovations, policy developments, and growth prospects in the excipients market.

A Position Paper from the AAPS In Vitro Release and Dissolution Focus Group

The financial and economic downturn is likey to have long-term implications for outsourcing.

GMP agents report on old products, aseptic violations, and unexpected emotions.
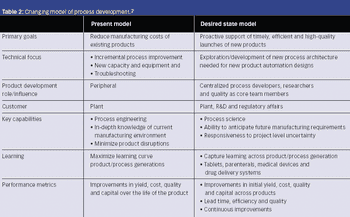
Operational excellence awaits, but only if you can implement PAT successfully.
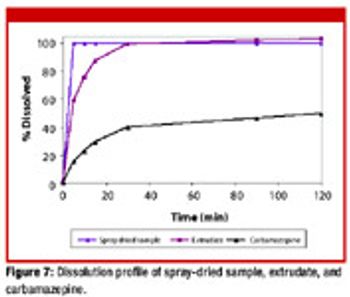
The authors demonstrate how melt-extrusion and spray-drying methods can help to prepare solid dispersions of poorly soluble drugs using Eudragit polymers.

The authors examine the use of a novel highly functional pregelatinized starch as a controlled-release matrix excipient.
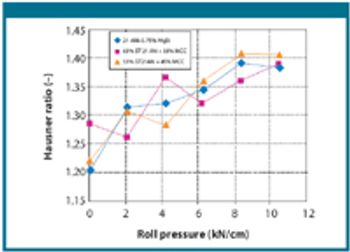
The authors studied the behavior of anhydrous lactose and the combination of anhydrous lactose and the combination of anhydrous lactose with microcrystalline cellulose on a pilot-scale roller compactor.
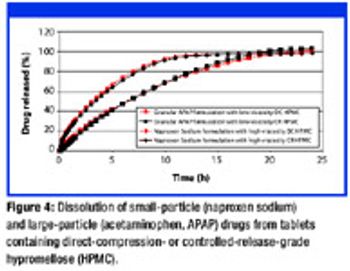
The authors examine the use of a hypromellose-based product as an excipient in a controlled release formulation using direct-compression tableting.

Also, Hospira to reduce workforce; WuXi AppTech makes senior appointments; more...

A bipartisan bill that would establish a regulatory pathway for the approval of biosimilars was introduced into the US House of Representatives last week.

The US Pharmacopeial Convention (USP) and the National Institute for the Control of Pharmaceutical Biological Products (NICPBP), China's agency for overseeing the quality of large- and small-molecule drugs, signed a memorandum of understanding (MOU) to bolster the quality of medicines in China and in the countries that buy Chinese drug products, including the United States.

Also, Genzyme receives warning letter; Mesa Laboratories appoints John J. Sullivan CEO and a member of the board of directors; more...

The spotlight on the biopharmaceutical industry is intensifying, as recently evidenced by Pfizer's (New York) ongoing acquisition of Wyeth (Madison, NJ), which was initiated partly to reduce the former's dependence on small-molecule drugs.

Also, Penn Pharma to expand; stem cell research funding ban lifted; Bristol-Myers Squibb made senior appointments; more...

Also, Schering-Plough's vaccine unit, Nobilon, formed an agreement with the World Health Organization; Ore Pharmaceuticals named president and CEO; more...

Scientists studying epilepsy have traditionally focused on the comings and goings of ions through molecular channels in nerve cells, and many current antiseizure therapies seek to modulate that dynamic.

Ultra high performance liquid chromatography is advantageous in a contract laboratory because it is faster, more sensitive, and relies on smaller volumes of organic solvents than HPLC.

USP's Stage 2 heparin monograph revisions address identification, potency, and impurities.
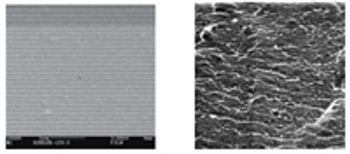
The authors investigate the effects of a polyethylene glycol plasticizer and water on cellulose acetate film properties.

Industry has changed, but its basic tenets have not. INTERPHEX's RJ Palermo discusses a 7-step process to keep pharma moving forward.

The source of a problem reveals itself after some investigation, or it may crash down on you.

Brief pharmaceutical news items for March 2009.

Contract manufacturers of APIs and intermediates report gains, but express caution.