
Recent advances in microreactor technology are improving the application and scale at which the technology can be applied.

Recent advances in microreactor technology are improving the application and scale at which the technology can be applied.

Company and People Notes: 3M forms agreement with VaxInnate; TRIN Pharma appoints CEO; More...

In response to a request from the FDA, the US Pharmacopeial Convention (USP) has revised its standards for propylene glycol and sorbitol solution, excipients widely used in prescription and over-the-counter drugs.

In response to a request from the US Food and Drug Administration, the US Pharmacopeial Convention (USP) revised its standards for propylene glycol and sorbitol solution

Company and People Notes: sanofi aventis forms pact with Micromet; Laureate Pharma adds members to its business team.

A panel of industry and regulatory experts, including FDA, discuss efforts to secure the global pharmaceutical supply chain. This article contains bonus online-exclusive material.

New tests solve one issue, but cheaper plastic and new stoppers cause problems.

While Congress debates hundreds of healthcare plan proposals, perhaps we, the public, can get in the game too.

Functionalized supramolecular catalysts and an enantioselective route to unnatural amino acids are some recent developments.

A recent book reminds readers that small-molecule chemistry has enabled advances in biotechnology.
The authors explain a process for moisture-activated dry granulation in detail and provide guidance for the selection of excipients and equipment.

FDA's Edwin Rivera-Martinez on dodging supply-chain challenges.
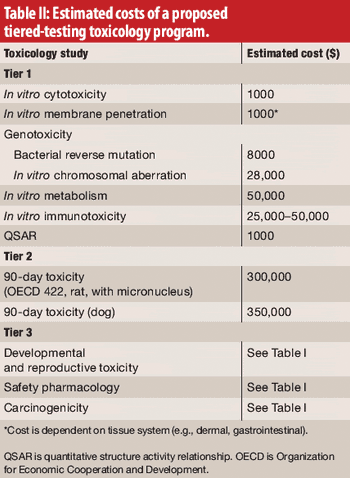
The authors, representing the International Pharmaceutical Excipients Council, propose a new evaluation procedure, including tiered toxicology testing for excipients.

The author describes how Merrion Pharmaceuticals reformulates parenteral drugs into tablets and capsules that are easier for patients to take and provide better bioavailability.

The author details the factors in formulation design, requirements in facilites and equipment, and validation criteria for aseptic formualtions.

A Conversation with Otonomy

Chronotherapeutic drug delivery systems (CRDDS) have been recognized as potentially beneficial to the chronotherapy (timeoptimized therapy) of widespread chronic diseases that display time-dependent symptoms.

Mark Copley discusses the methods used for DPI testing and the challenges presented by the current regulatory framework.

An inhaler mouthpiece that optimizes drug delivery to the lungs and reduces the amount of wasted medication has been developed by US researchers.

Also, Astellas and Medivation sign development pact; WuXi PharmaTech appoints VP of business development, more...

The global API market has expanded from $69 billion in 2004 to more than $90 billion in 2008, according to a report from the Italian Chemical Pharmaceutical Association (CPA); in particular, the report noted a rapid growth rate for generic APIs.

Company and People Notes: SurModics forms agreement with Roche and Genentech; Hospira names Daphne Jones senior VP and chief information officer; more...

The European Fine Chemicals Group (EFCG) gave an update of some of its activities at a press conference held at CPhI Worldwide (Spain) last week, and revealed some disturbing facts regarding counterfeit APIs, which the group is particularly concerned about.

The manufacturing process, which influences a drug's safety and efficacy, is particularly critical for drugs administered through injection, and personnel must closely supervise lyophilization to ensure product quality.

ISP Pharmaceuticals is embarking on a Drug Solubility Initiative to support pharma companies working with poorly soluble drug actives.